GROUPS IN THE PERIODIC TABLE
A GCSE chemistry revision page on groups in the periodic table covering the alkali metals’ properties and reactivity trends, noble gas inertness and uses, halogen states and reactivity patterns, reactions with metals, and displacement trends down group 7.
CH181: Identify the groups on the Periodic Table
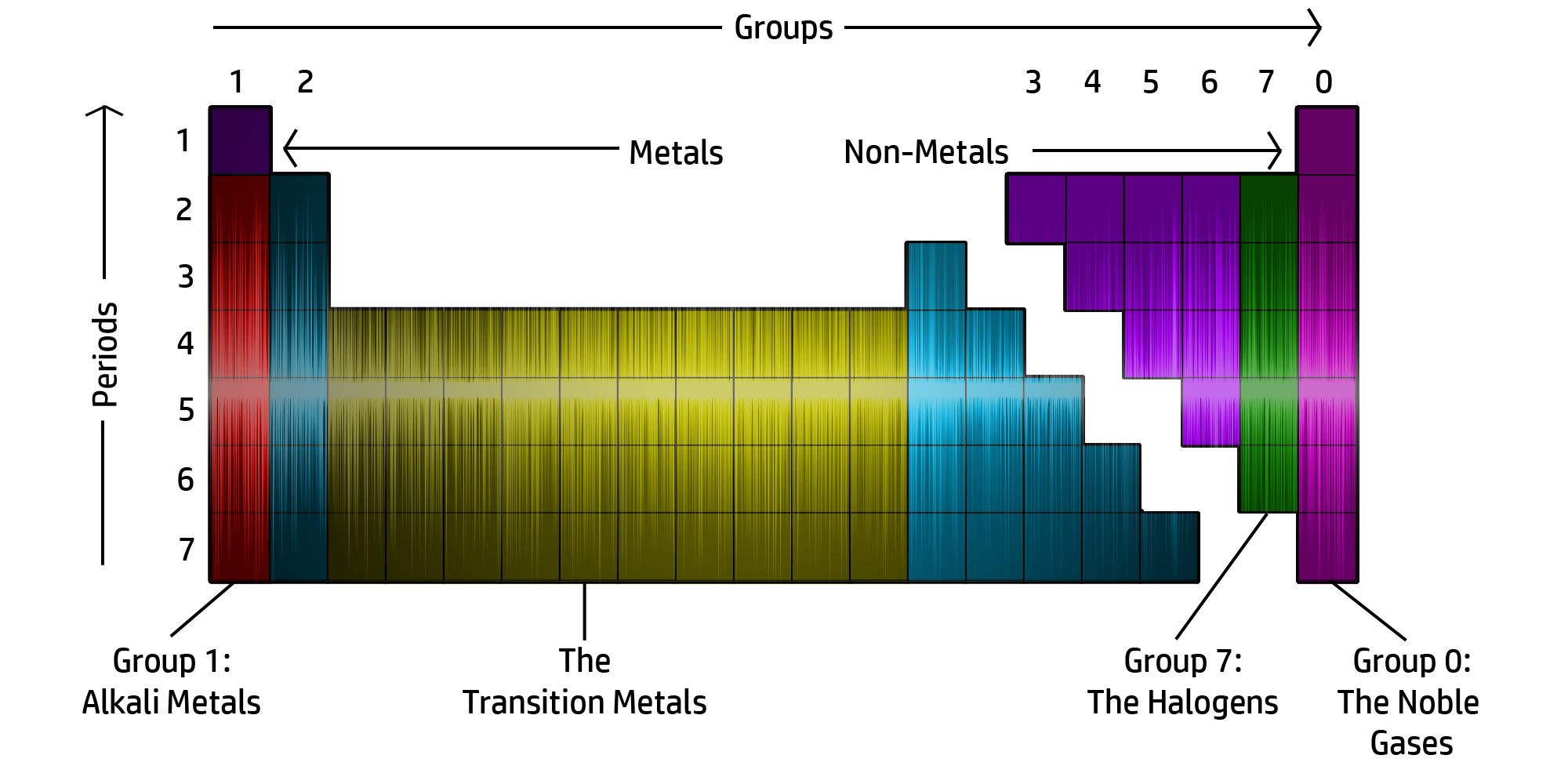
CH182: Describe the physical properties of the alkali metals
Alkali metals have similar physical properties to most metals (CH71), but there are some differences:
- They have much lower melting points than most metals.
- They are very soft – can be cut easily.
- They have low densities (lithium, sodium and potassium can float on water)
CH183: Write word and chemical equations for reactions between alkali metals and water
When alkali metals are added to water, they all react the same way. They all react with water to produce a hydroxide and hydrogen gas.
The word and balanced chemical equations are identical:
Potassium + Water → Potassium Hydroxide + Hydrogen
2K (s) + H2O (l) → 2KOH (aq) + H2 (g)
You can write word and balanced chemical equations easily for the alkali metals and water by just replacing the name/symbol for potassium above with the name/symbol of the alkali metal. For more information on alkali metal word equations see CH4 and for balanced equations check CH13.
CH184: Describe the similarities and differences when the alkali metals are added to water
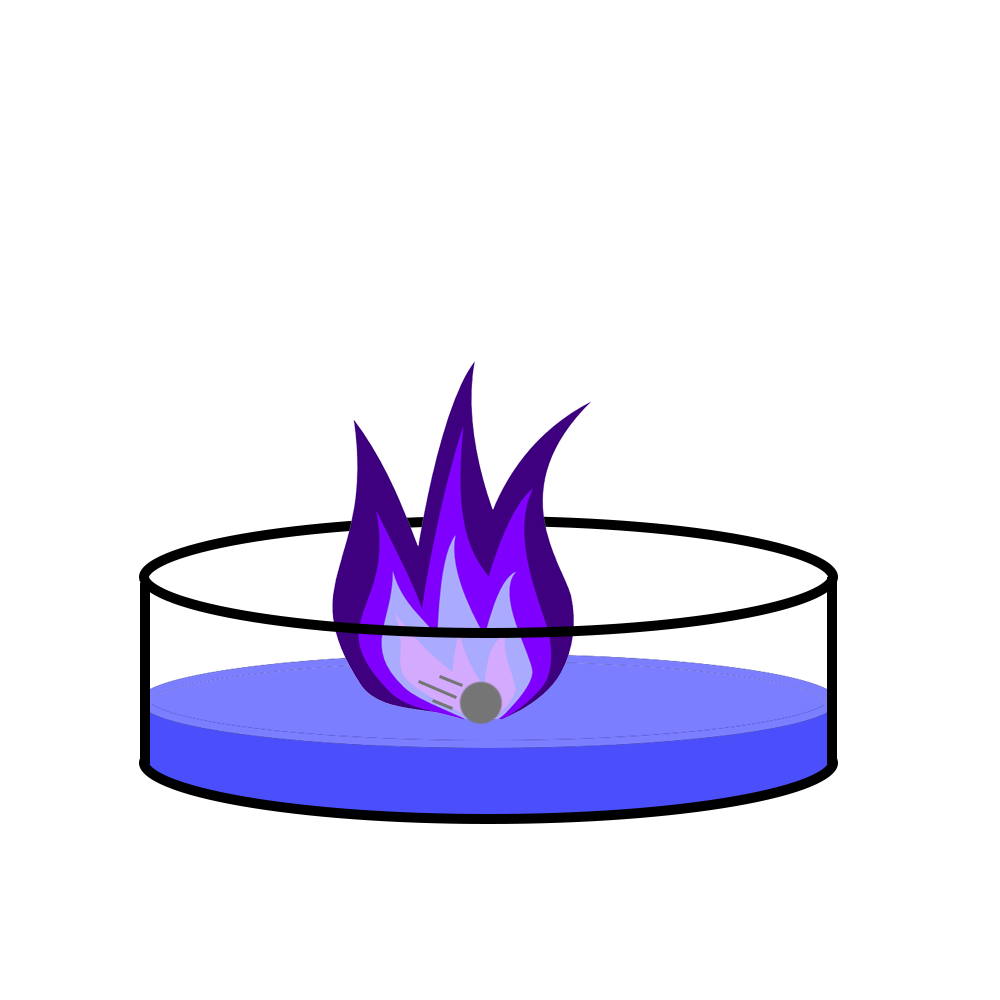
When the alkali metals are added to water, you need to remember the similarities:
- They all float on water.
- They all move on water.
- They all fizz.
As you go down the group (Lithium → Sodium → Potassium), the reactivity increases. We can see this because:
- Lithium only moves and fizzes a small amount.
- Sodium fizzes more, moves slightly faster than lithium, and turns into a molten ball.
- Potassium fizzes the most, sets on fire with a lilac flame, and can explode.
You can use this to predict the properties of the rest of the alkali metals – as you go down, the move more, fizz more and will most likely explode!
CH185: Explain why reactivity increases as you go down group one
As you go down group 1, reactivity increases. This is all to do with electronic configuration:
- As you go down the group, the atom has more shells of electrons.
- There is more electron shielding.
- The outer electron is further away from the nucleus.
- The force of attraction between the outer electron and the positive nucleus is weaker.
- It is easier to lose the outer electron – therefore more reactive!
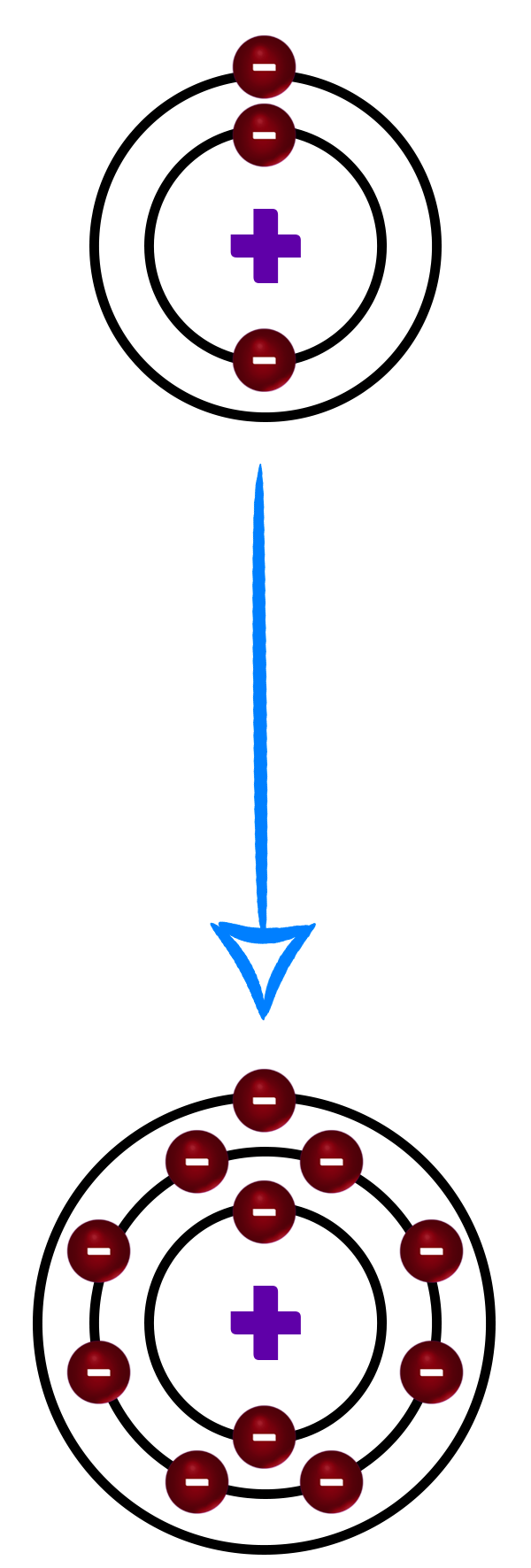
CH186: Explain why the noble gases are inert

The Noble Gases are found in group 0 of the Periodic table and contain helium, neon, argon, krypton, xenon, and radon.
They are in group 0 because they all have full outer shells.
This makes all noble gases inert.
This means that they are unreactive – they don’t need to lose or gain any electrons to get a full outer shell.
They are all monatomic – which means they go around on their own.
CH187: Describe the uses and properties of the noble gases

Argon, Xenon and Krypton can be used in lightbulbs.
Argon is used in filament light bulbs
Krypton and Xenon are used in flash photography.
Explanation: Argon has a full outer shell, so doesn't want to lose / gain / share any electrons. Therefore, it will not react with the hot filament (unlike oxygen), stopping it from breaking.
Argon is also used in welding.
Explanation: Argon is denser than air, so it stops oxygen from getting to the metal. It has a full outer shell, so doesn't want to lose / gain / share any electrons. Therefore, it will not react with the metal (unlike oxygen), stopping it from getting impurities.


Argon is also used in wine barrels
Explanation: Argon is placed in the top of wine barrels, replacing oxygen from the air. It has a full outer shell, so doesn't want to lose / gain / share any electrons. Therefore, it will not react with the wine (unlike oxygen), stopping it from oxidising and turning into a carboxylic acid.
Helium is used in airships, weather balloons and party balloons
Explanation: Helium is less dense than air, so will float. It is used instead of hydrogen because it has a full outer shell, so doesn't want to lose / gain / share any electrons. Therefore, it will not set on fire (unlike hydrogen), stopping it from exploding

CH188: Describe the trend in physical properties for the noble gases
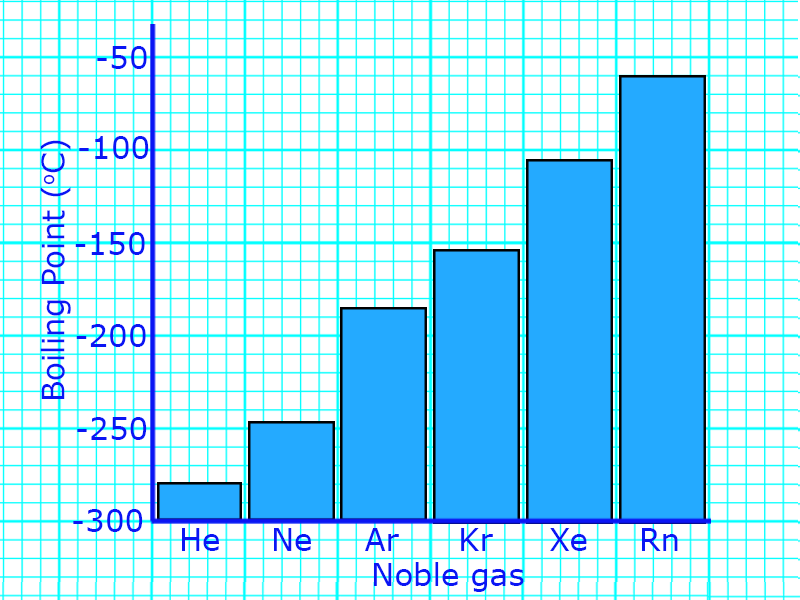
The noble gases all seem similar, but the physical properties do change as you go down the group:
- Boiling point and Melting point increases down the group
- Density increases as you go down the group
You can use the information about these densities to predict the properties of different noble gases.
CH189: Identify the state and colour of the halogens
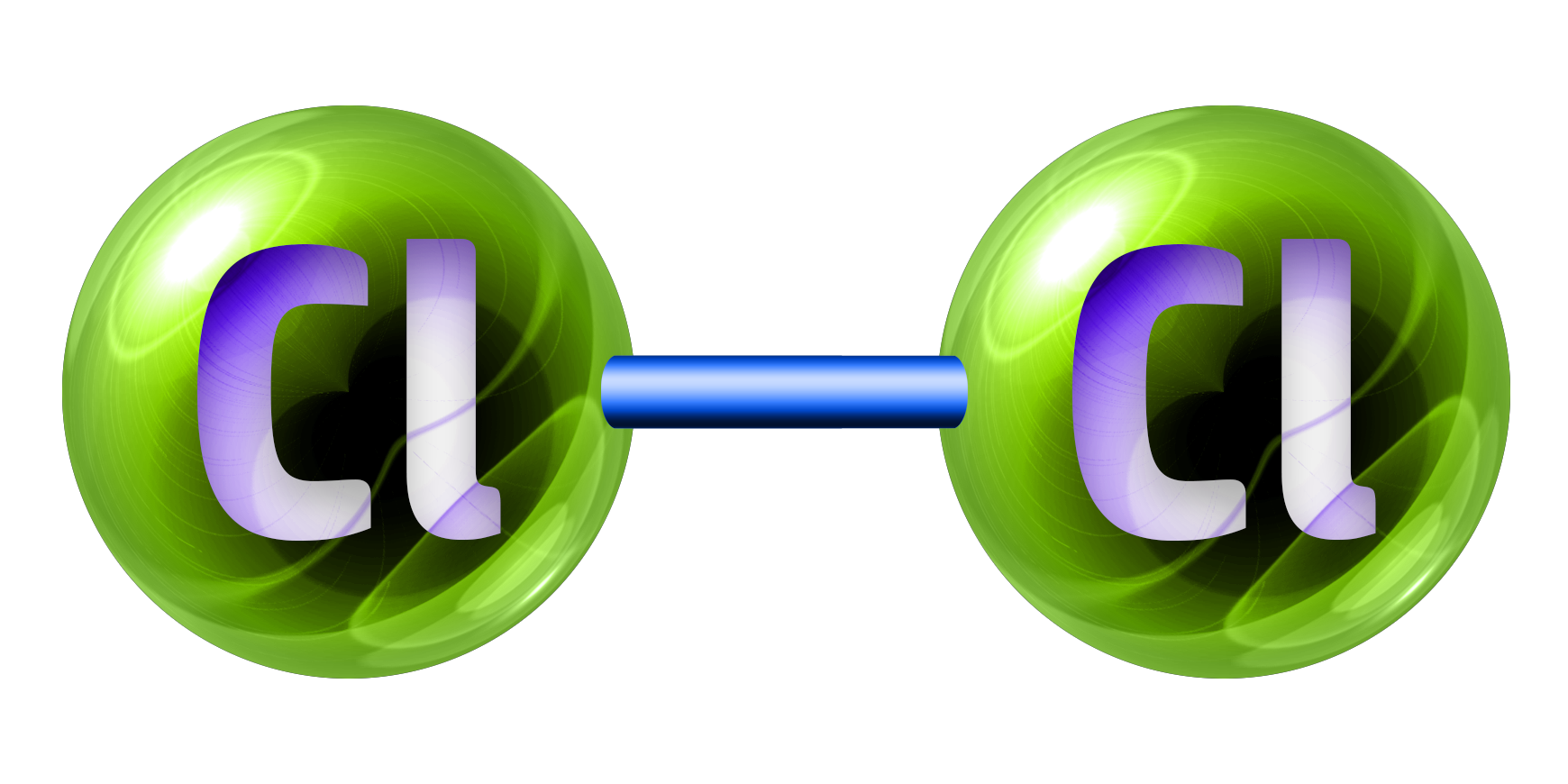
Chlorine, Cl2, is a green gas.
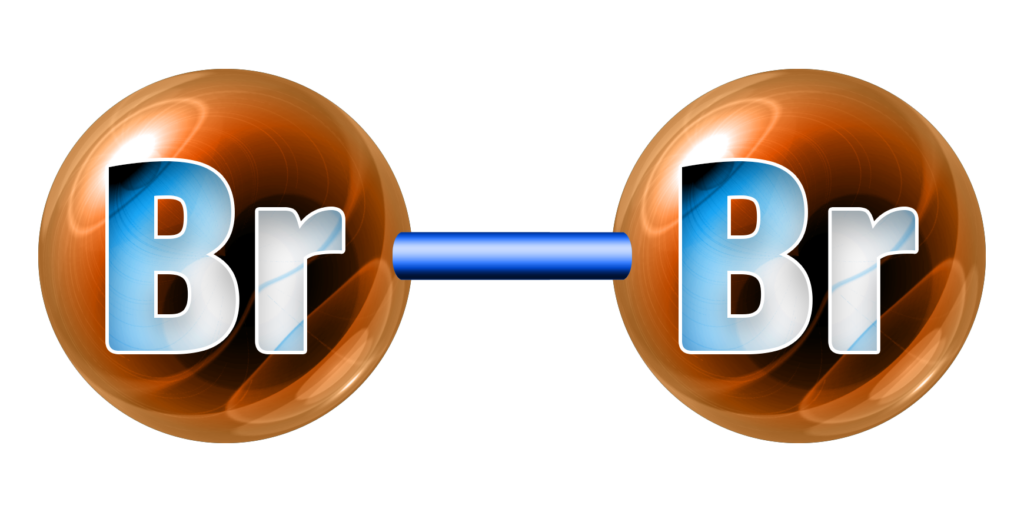
Bromine, Br2, is a red-brown liquid.
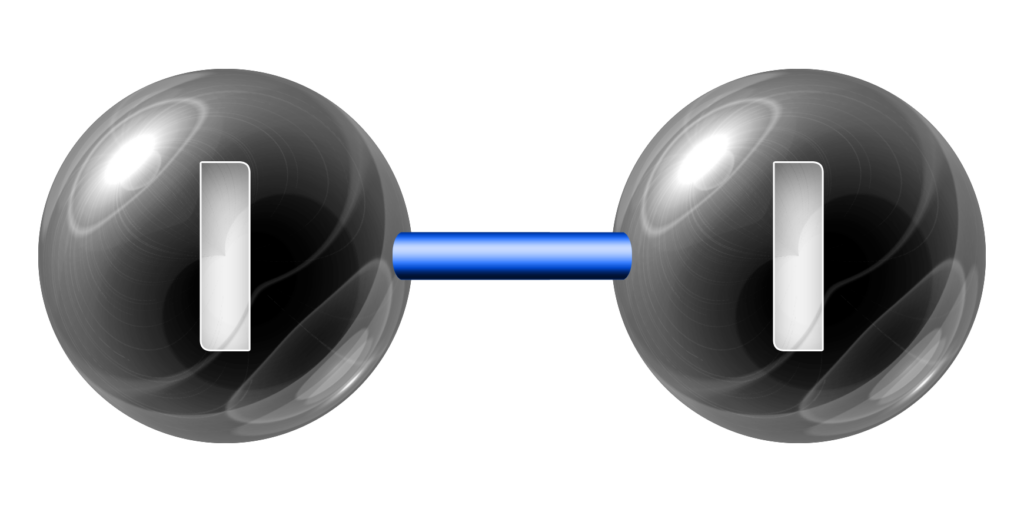
Iodine, I2, is a black solid.
CH190: Describe the trend in properties of the halogens
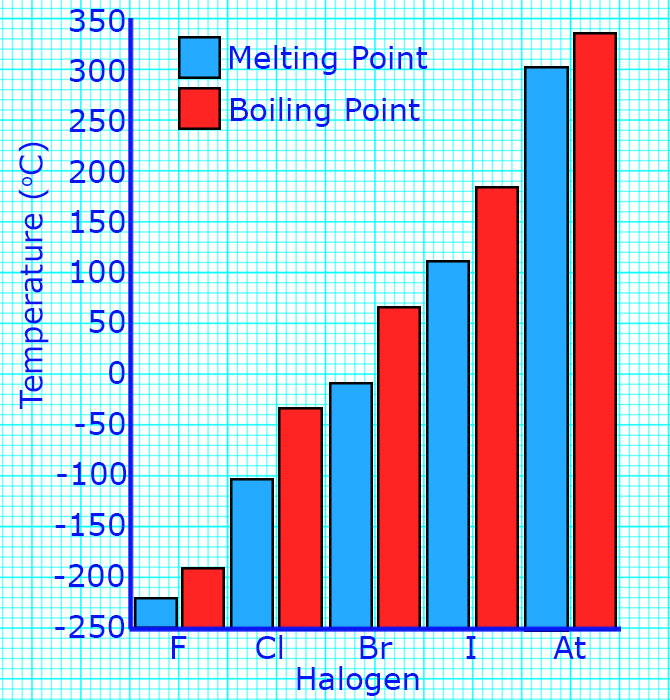
As you go down group 7, the melting points and boiling points increase.
You can tell this because the states change from gases to solids as you go down the group.
CH191: Describe the test for chlorine
If you wanted to prove that a gas was chlorine, add damp blue litmus paper to it.
Damp blue litmus paper turns red, then bleaches.
CH192: Describe the reactions of the halogens with metals
When the halogens react with metals, they form metal halides. The reactions are all similar, depending on what group you are reacting with:
- Group 1 metals give the general formula of M1H1 – one metal and one halogen.
- Group 2 metals give the general formula of M1H2 – one metal and two halogens.
- Group 3 metals give the general formula of M1H3 – one metal and three halogens.
Group 1 Example: Write the word and balanced equation for the reaction between sodium and iodine.
Sodium + Iodine → Sodium Iodide
2Na (s) + I2 (s) → 2NaI (s)
Group 2 Example: Write the word and balanced equation for the reaction between magnesium and bromine.
Magnesium + Bromine → Magnesium Bromide
Mg (s) + Br2 (l) → MgBr2 (s)
Group 3 Example: Write the word and balanced equation for the reaction between aluminium and chlorine.
Aluminium + Chlorine → Aluminium Chloride
2Al (s) + Cl2 (g) → 2AlCl3 (s)
CH193: Describe the reactions of the halogens with hydrogen
When the halogens react with hydrogen, the same reaction occurs - hydrogen halides are formed.
Example: Write the word and balanced equation for the reaction between hydrogen and chlorine.
Hydrogen + Chlorine → Hydrogen Chloride
H2 (g) + Cl2 (g) → 2HCl (g)
All the hydrogen halides are gases, and when they dissolve into water, they form an aqueous solution which is acidic.
- Hydrogen chloride, HCl (g), dissolves to form hydrochloric acid, HCl (aq).
- The hydrogen ion, H+, is what makes the solution acidic.
CH194: Investigate the reactivity of the halogens
As you go down group 7, reactivity decreases. This can be proved using displacement reactions – where the more reactive halogen will always end up in the salt.
If you take a salt containing chlorine, bromine and iodine, and react them with solutions of their elements (Cl2, Br2 and I2), the following will occur:
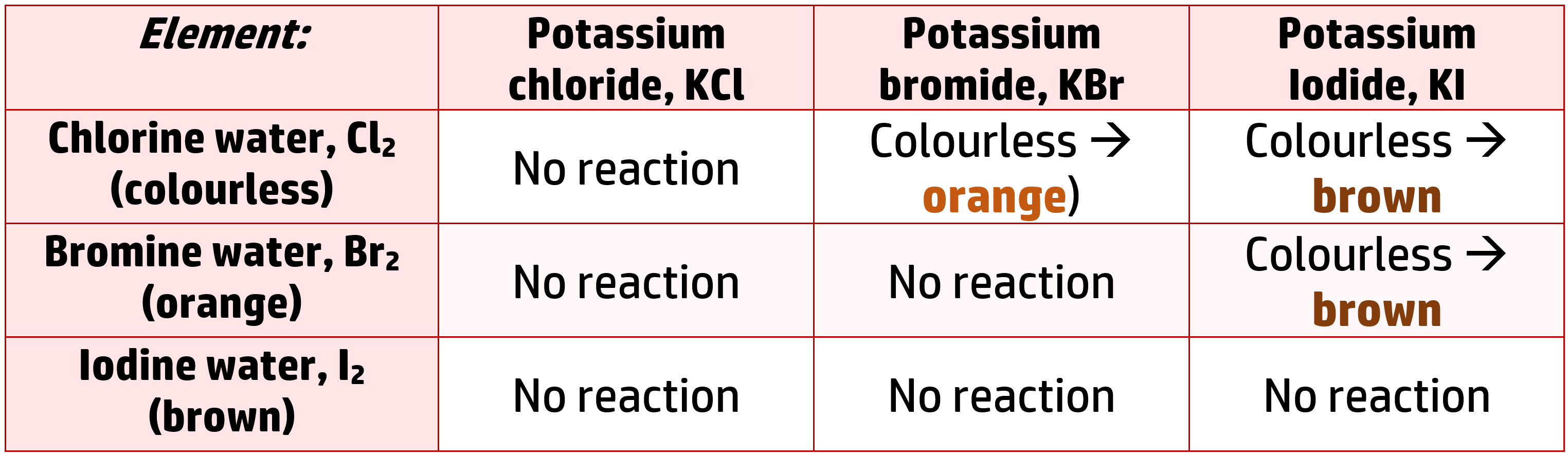
This shows us that chlorine is more reactive than both bromine and iodine because it displaces them both to form potassium chloride.
It also shows that bromine is more reactive than iodine because it displaces it (you see a reaction), but it is less reactive than chlorine (no reaction occurs)
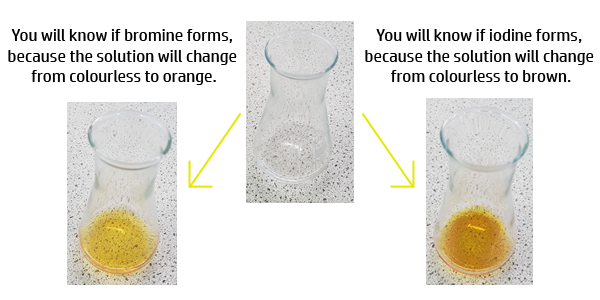
Example of a displacement reaction:
Chlorine + Potassium Bromide → Potassium Chloride + Bromine
Cl2 + 2KBr → 2KCl + Br2
CH195: Link displacement reactions of the halogens to REDOX reactions
The displacement reactions seen in CH194 are all examples of REDOX reactions.
In the above reaction, where chlorine displaces the bromine in potassium bromide, the following reaction occurs:
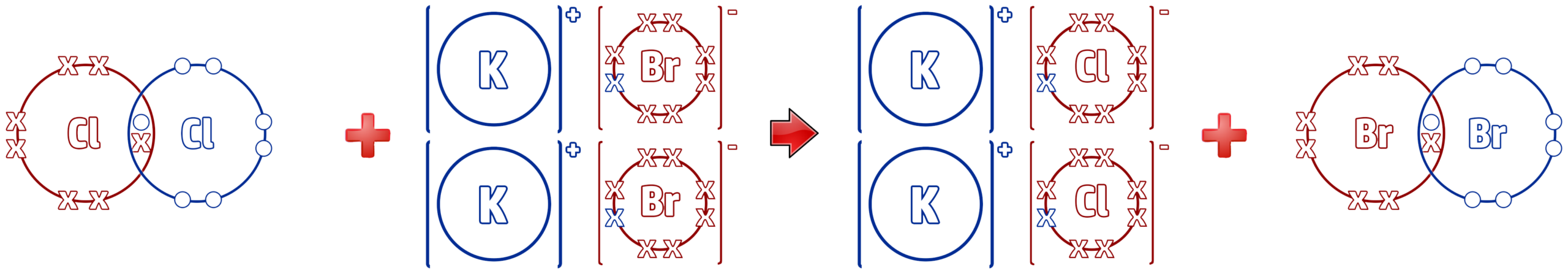
As you can see from the dot and cross diagram above, the chlorine molecule has 14 electrons on the left and the chlorine ions have 16 electrons on the right.
This means that chlorine has gained electrons – RIG – Reduction Is Gain. Chlorine is reduced.
The opposite happens to bromine – OIL – Oxidation Is Loss of electrons. The halogen (e.g. Cl2) always gains electrons (reduced) and the ionic halide (e.g. 2KBr) is always oxidised, as they lose electrons.
CH196: Explain why reactivity of the halogens decreases as you go down group 7

As you go down the group, the atom has more shells of electrons.
There is more electron shielding.
The outer electron is further away from the nucleus.
The force of attraction between the outer electron and the positive nucleus is weaker.
It is harder to gain the outer electron – therefore less reactive!
