ELECTROLYSIS

This page is an Edexcel GCSE revision page explaining electrolysis, including definitions of electrolytes and electrolysis, ion movement at electrodes, making pure copper, the electrolysis core practical with variables and risks, predicting products, and writing half-equations.
CH133: Describe what an electrolyte is

An electrolyte is any liquid that contains ions (atoms that have gained or lost electrons to become charged particles).
The liquid can be molten (melted) or aqueous (dissolved).

CH134: Describe what electrolysis is
Electrolysis is the breaking down of an electrolyte (CH133) using electrical energy.
The electrical energy needed is direct current.
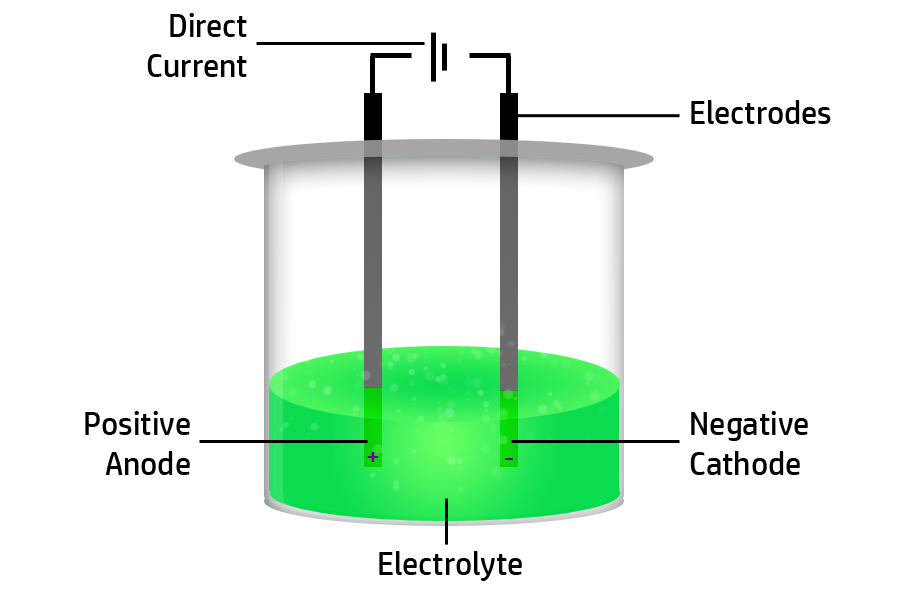
CH135: Describe the movement of ions during electrolysis
Firstly, remember that opposites attract – so positive ions will move to the negative electrode and negative ions will move to the positive electrode.
Secondly, remember the names of the electrodes by remembering the Mnemonic on the right: Positive Anode, Negative Is Cathode …so remember to PANIC in your exam!
Therefore, positive cations (paw-sitive!) will move to the negative cathode and negative anions will move to the positive anode

CH136: Describe how pure copper can be made using electrolysis
If you want to make copper pure, you can use electrolysis.
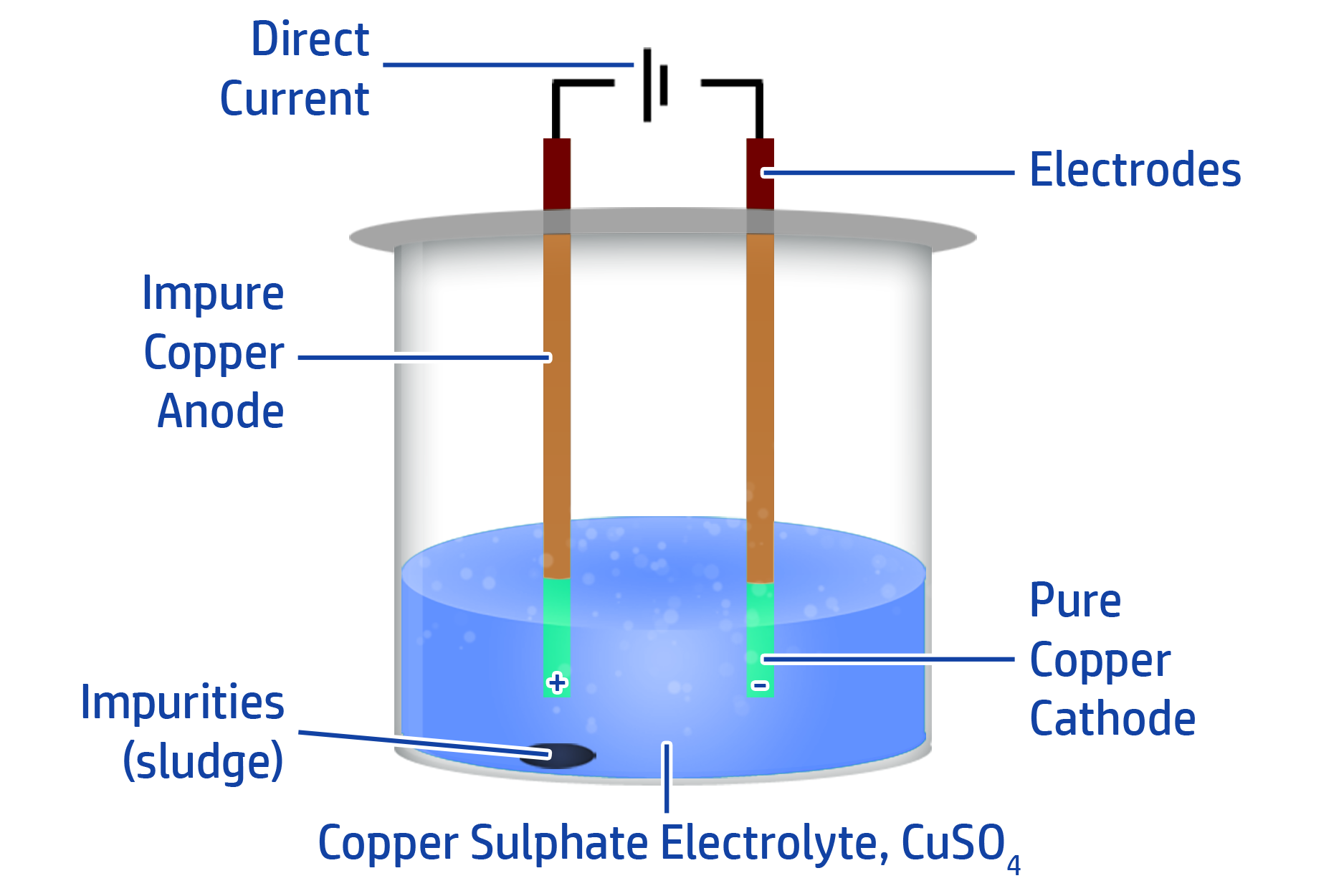
To do this, add your impure copper to the positive anode and a pure copper electrode onto the negative cathode.
You then need to have a solution that contains copper ions as your electrolyte (such as copper sulfate).
When you turn the D.C power supply on, the copper from the impure electrode will turn into copper cations (Cu2+). They will then move towards the negative cathode and turn back into copper atoms.
This removes the copper from the impure electrode and adds it to the pure electrode. You will be left with sludge in the solution – which is the ‘dirt’ from your impure electrode.
CH137: Core Practical: Investigating the electrolysis of copper sulfate

Electrolysis of CuSO4 with copper electrodes:
Why does the mass of the anode decrease?
- During the electrolysis, the copper atoms in the impure copper anode lose two electrons and turn into Cu2+ ions (oxidation).
- (H) Anode half equation (see CH139): Cu → Cu2+ + 2e-
Why does the mass of the anode decrease?
- The copper cations in the electrolyte move to the cathode and gain two electrons (reduction) to turn back into copper atoms.
- (H) Cathode half equation (see CH139): Cu2+ + 2e- → Cu
Why is the change in mass different?
- The anode mass will decrease more than the cathode mass will increase.
- This is because there are impurities on the anode that fall off during electrolysis.
- The impurities will fall to the bottom of the beaker as sludge.
Method:
Key Steps: Mass → Variable Resistor → Electrolyte → Wash → Dry → Mass
You will need to be able to explain how to investigate the change of current on the mass of the electrodes in the above set up.
- Measure the mass of the cathode and anode.
- Use a variable resistor to set the current to 0.2A.
- Add the electrodes to the electrolyte and leave for 20 minutes.
- Wash the electrodes to remove impurities
- Dry the electrodes by either gently patting them or dipping them in propanone. Do not scrub them or the copper will be wiped off.
- Measure the final mass of the electrodes and calculate the change in mass.
What happens if we use Inert Electrodes?
If you carry out electrolysis of copper sulphate using inert (unreactive) electrodes instead of copper electrodes, you see different things:
- The cathode will be coated in copper:
- Half equation (H) (see CH139): Cu2+ + 2e- → Cu
- Oxygen will form at the anode, which will be seen as bubbles:
- Half equation (H) (see CH139): 4OH- → O2 + 2H2O + 4e-
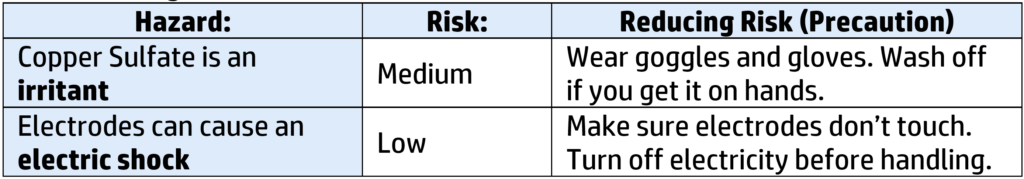
Risks and Management:
Variables:
- Independent variable (the thing you change!): The current
- Dependent variable (the thing you measure!): The change in mass of the electrodes.
- Control variables (the things you keep the same!): The volume of copper sulfate, the concentration of copper sulfate, how far the electrodes are dipped into the copper sulfate.
CH138: Predicting the products of electrolysis
Molten electrolytes: Example – Lead Bromide, PbBr2 (l)
- When you have a molten electrolyte, you only have two ions in the liquid – the metal and the non-metal.
- At the cathode, lead ions (Pb2+) will gain electrons (reduction) and turn back into lead atoms.
- At the anode, bromide ions will lose electrons (oxidation) and turn back into bromine molecules, Br2.
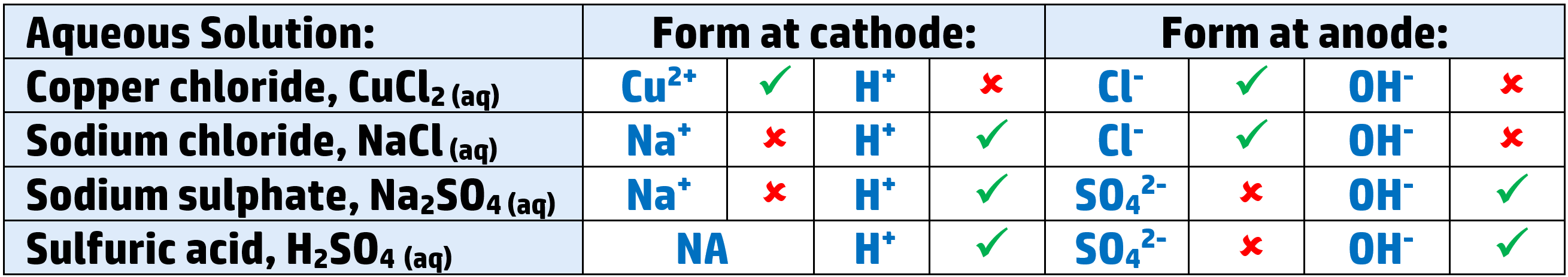
Aqueous electrolytes:
- When you electrolyse an aqueous solution, you have H+ and OH- ions as well as the metal and non-metal ions.
- For example: copper chloride, CuCl2, will have Cu2+ and H+ cations as well as Cl- and OH- anions.
- You need to be able to work out what will form. There are two rules to know:
- Cathode: The least reactive ion will form.
- Anode: If there is a halide (group 7), that will form – if not, the hydroxide will go there, and form water and bubbles of oxygen.
CH139: Writing Half Equations for electrolysis (H)
Once you know what forms at each electrode (CH138), you need to be able to write half equations. This involves looking at the change in electrons on each side.
Example: When molten zinc chloride is electrolysed, zinc ions, Zn2+, form zinc atoms. Write the half equation for this reaction.
Step 1: Write out the start of your half equation. The question tells you that the zinc ions are turning into zinc atoms, so it goes in this order: Zn2+ → Zn
Step 2: Work out what is happening to the electrons. Zn2+ has a full outer shell and is going back to its natural atom. To do this it needs to gain electrons back (it had lost 2 to become positively charged).
It will gain two electrons to get from Zn2+ to Zn – which is written like this:

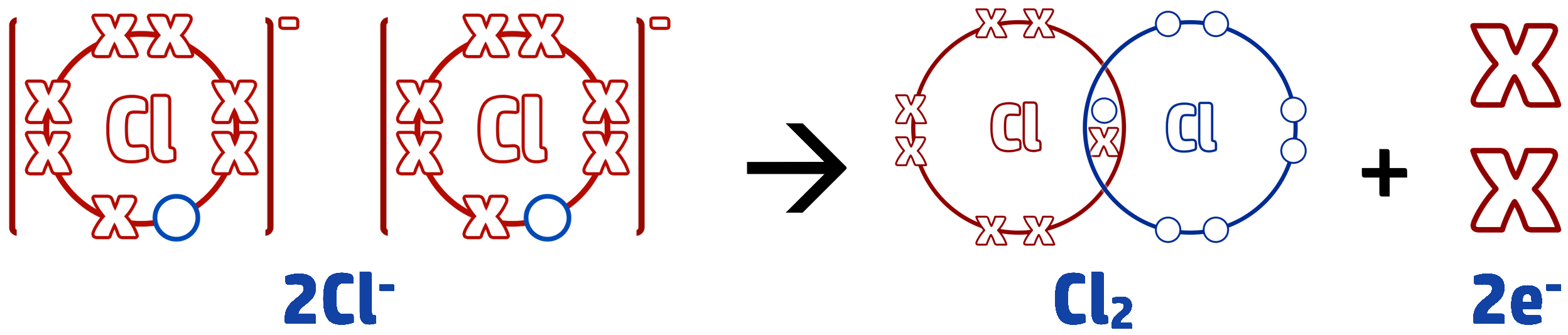
Example 2: When molten zinc chloride is electrolysed, chloride ions, Cl-, form chlorine molecules, Cl2. Write the half equation for this reaction.
Step 1: Write out the start of your half equation. The question tells you that the copper ions are turning into copper molecules, so it goes in this order: Cl- → Cl2
Step 2: Work out what is happening to the electrons. Cl- has a full outer shell and is going back to a molecule. To do this each chloride ion needs to lose an electron (they had gained 1 to become negatively charged). We write the loss of electrons on the right side of the arrow: Cl- → Cl2 + e
Step 3: We need to balance the half equation. To form Cl2, we need 2 chloride ions. Therefore, both of the chloride ions will need to lose an electron, so the balanced equation looks like this:
CH140: Electrolysis and REDOX reactions: Oxidation and Reduction (H)
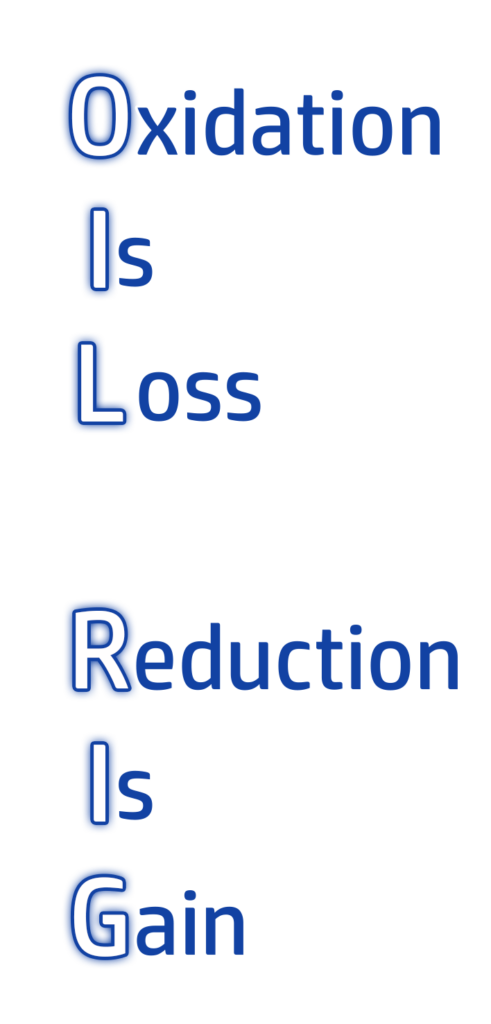
A REDOX reaction is any reaction that involves reduction and oxidation.
You may have come across oxidation when talking about the addition of oxygen, and reduction as the loss of oxygen, but there is another definition involving electrons:
- Oxidation is the loss of electrons.
- Reduction is the gain of electrons.
The order can be remembered by using the mnemonic OIL RIG (see left!)
CH141: Using REDOX to explain what happens at the electrodes (H)
When an ion goes back to its atom during electrolysis, it will either gain or lose electrons.
In the electrolysis of molten sodium chloride:
- The sodium (Na+) ions gain electrons to turn back into sodium metal. Reduction is occurring (see CH140).
- The chloride (Cl-) ions lose electrons and turn back into chlorine gas, Cl2. Oxidation is occurring (see CH140).


