Covalent Bonding
Simple Molecular

Giant Covalent
GCSE revision page on covalent bonding covering what covalent bonds and molecules are, drawing dot-and-cross diagrams using valency, simple molecular properties (melting/boiling, conductivity), giant covalent structures, diamond vs graphite, and graphene/fullerenes/nanotubes.
CH58: Describe what a covalent bond is
A covalent bond is a shared pair of electrons between non-metals.
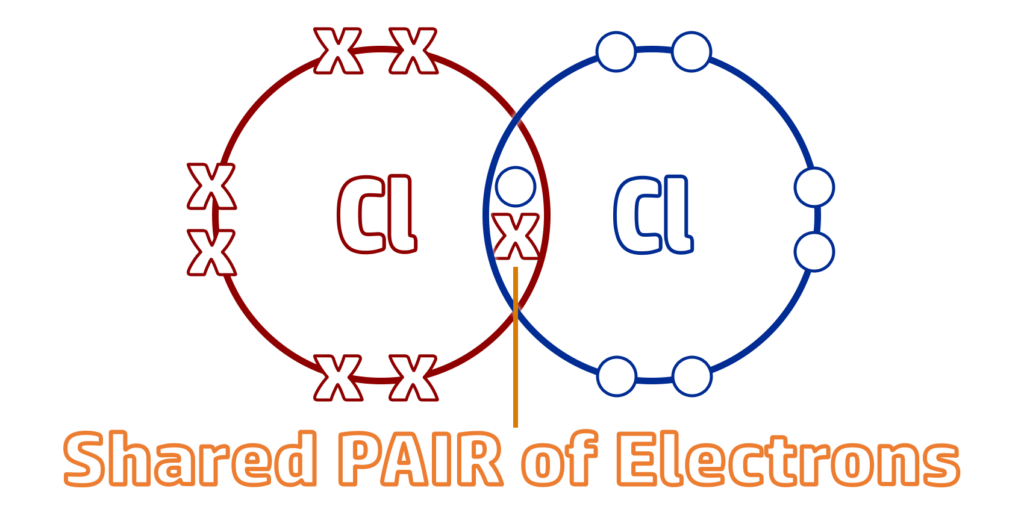
CH59: Describe what a molecule is
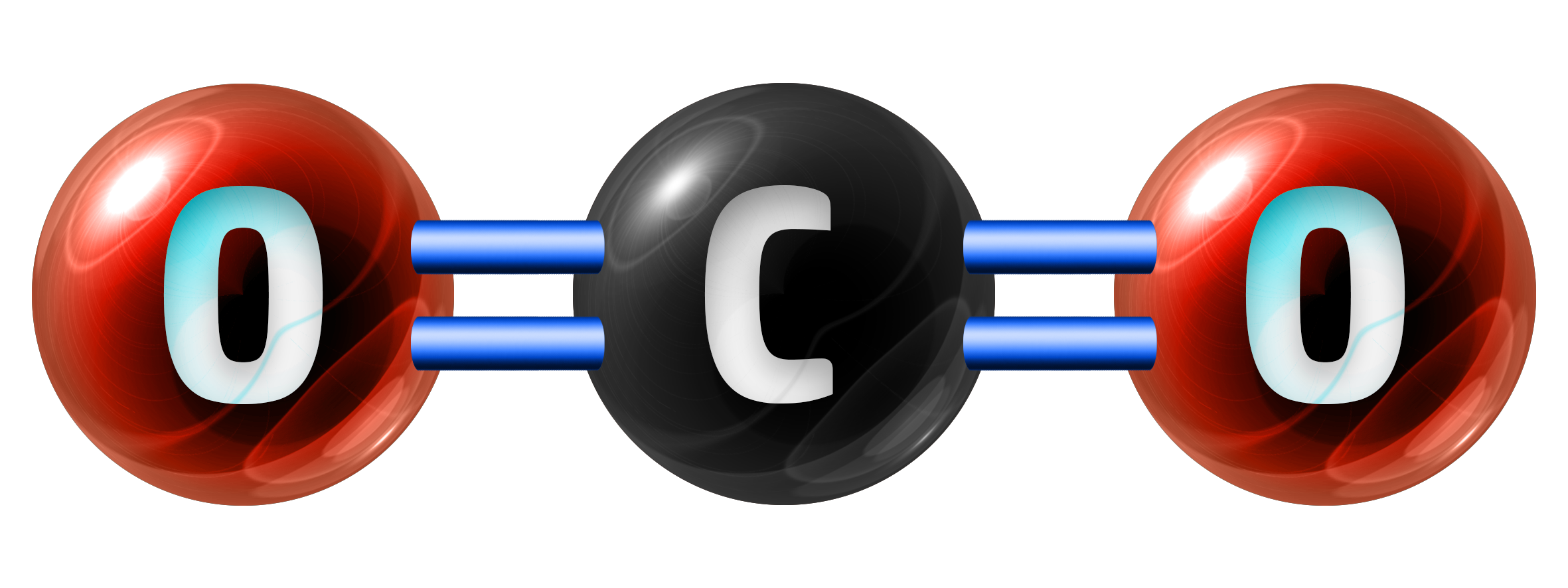
A molecule is a substance that involves covalent bonding and only contains a few atoms. Carbon dioxide, CO2, is an example of a molecule.
CH60: Identify the size of a molecule
Molecules are tiny, usually around 1-100nm small.
To convert nm into metres, multiply the size in nm by 1x10-9. To convert metres into nm, multiply the size in m by 1x109
CH61: Draw dot and cross diagrams for covalent substances
Step 1: Work out the valency – this is the number of electrons an atom needs to lose or gain to get a full outer shell.
On the right, chlorine (in group 7) has 7 electrons in its outer shell. It wants to gain one electron, so has a valency of 1: Cl1
Oxygen (in group 6) has 6 electrons in its outer shell. It wants to gain two electrons, so has a valency of 2: O2

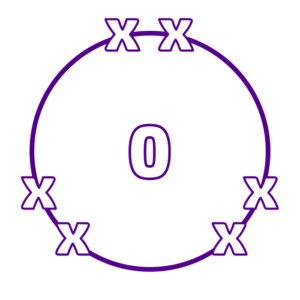
Step 2: The valency is the number of bonds an atom can make. So oxygen can make two bonds and chlorine can make 1.
This means that we will need two chlorines to react with one oxygen, giving us the formula OCl2

Step 3: Redraw the stick diagram, but with a circle overlap for every ‘stick’:
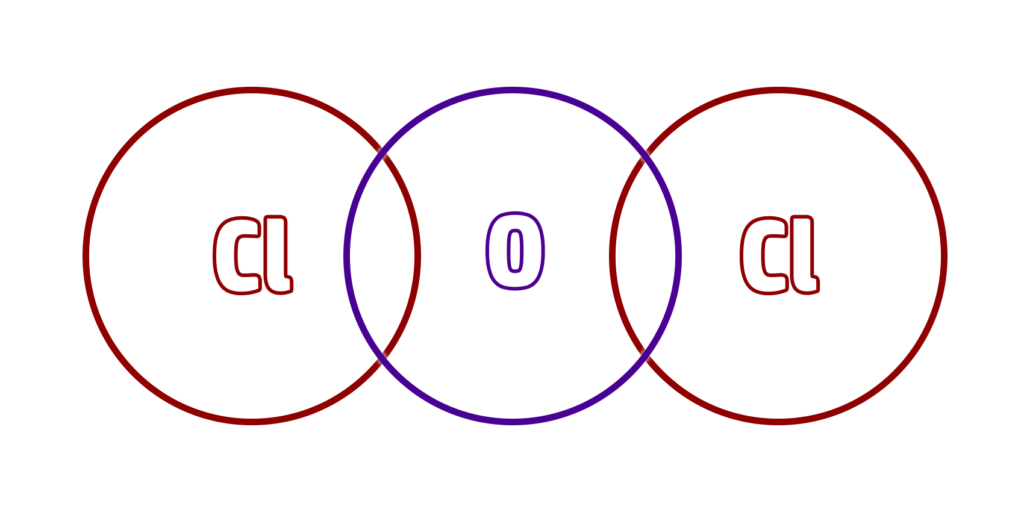
Step 4: Put a shared pair of electrons in the overlaps (a dot and cross).
This shared pair of electrons is your covalent bond.
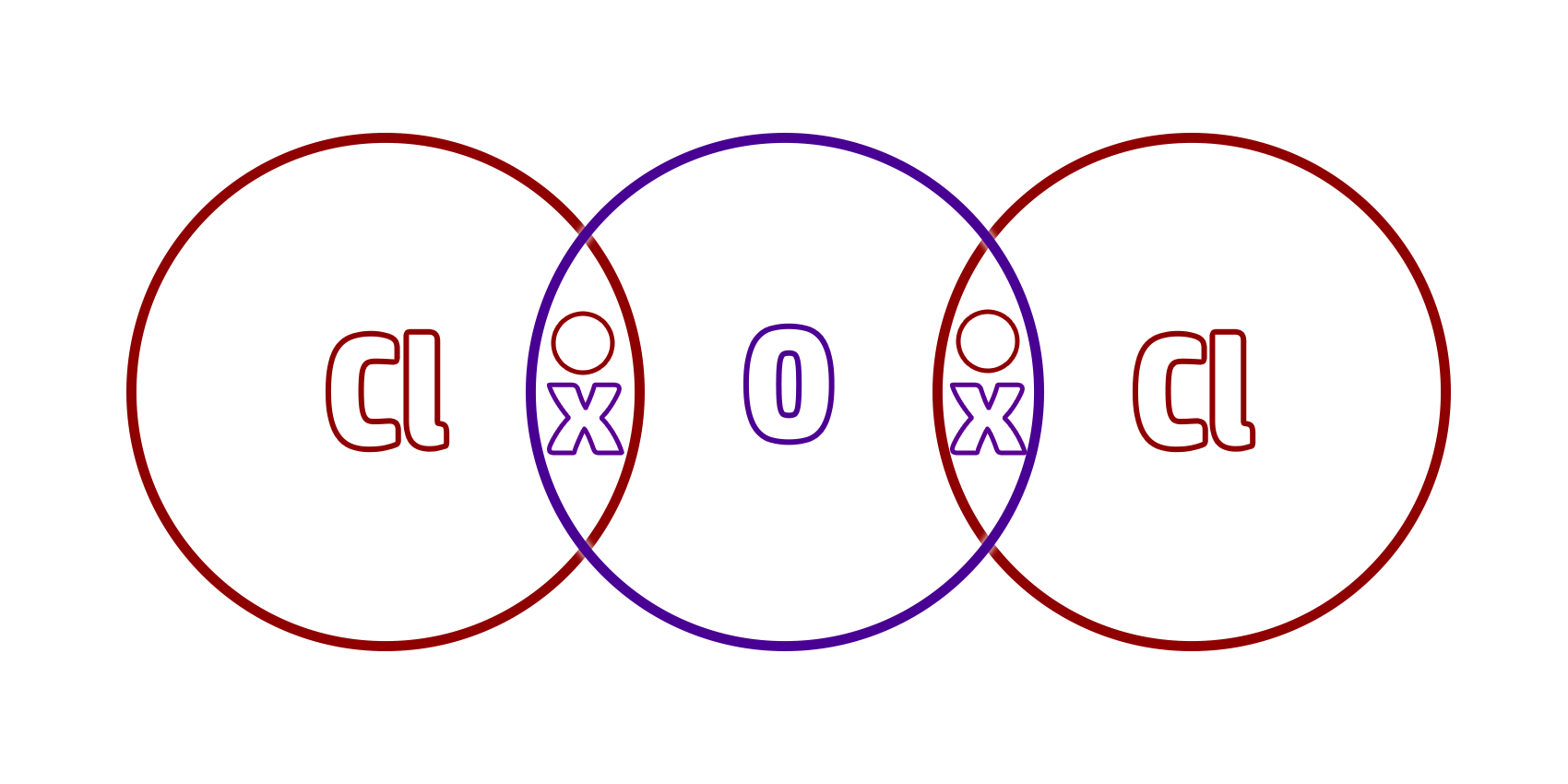
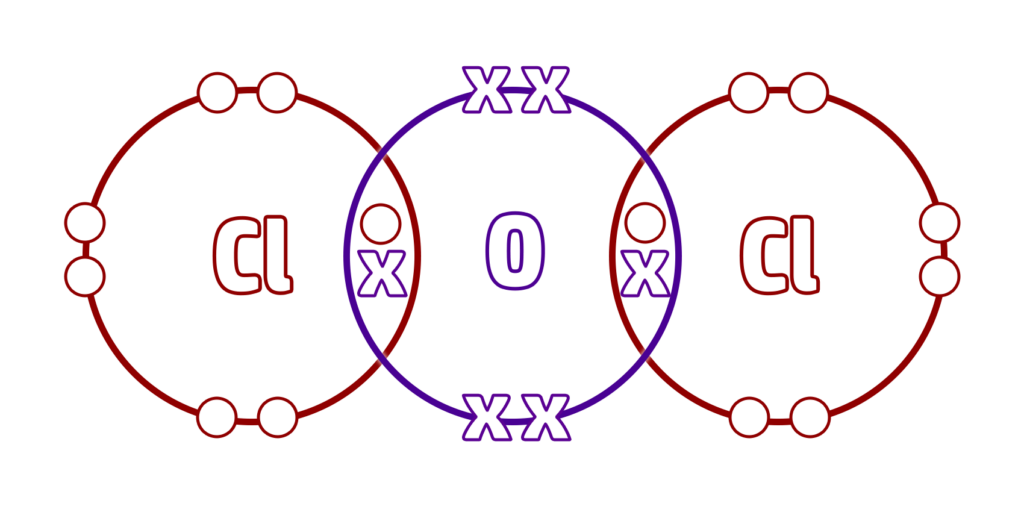
Step 5: Make sure that each atom has a full outer shell (everything should have 8 electrons except for hydrogen which should only have 2)
Watch the video on the right for more examples.
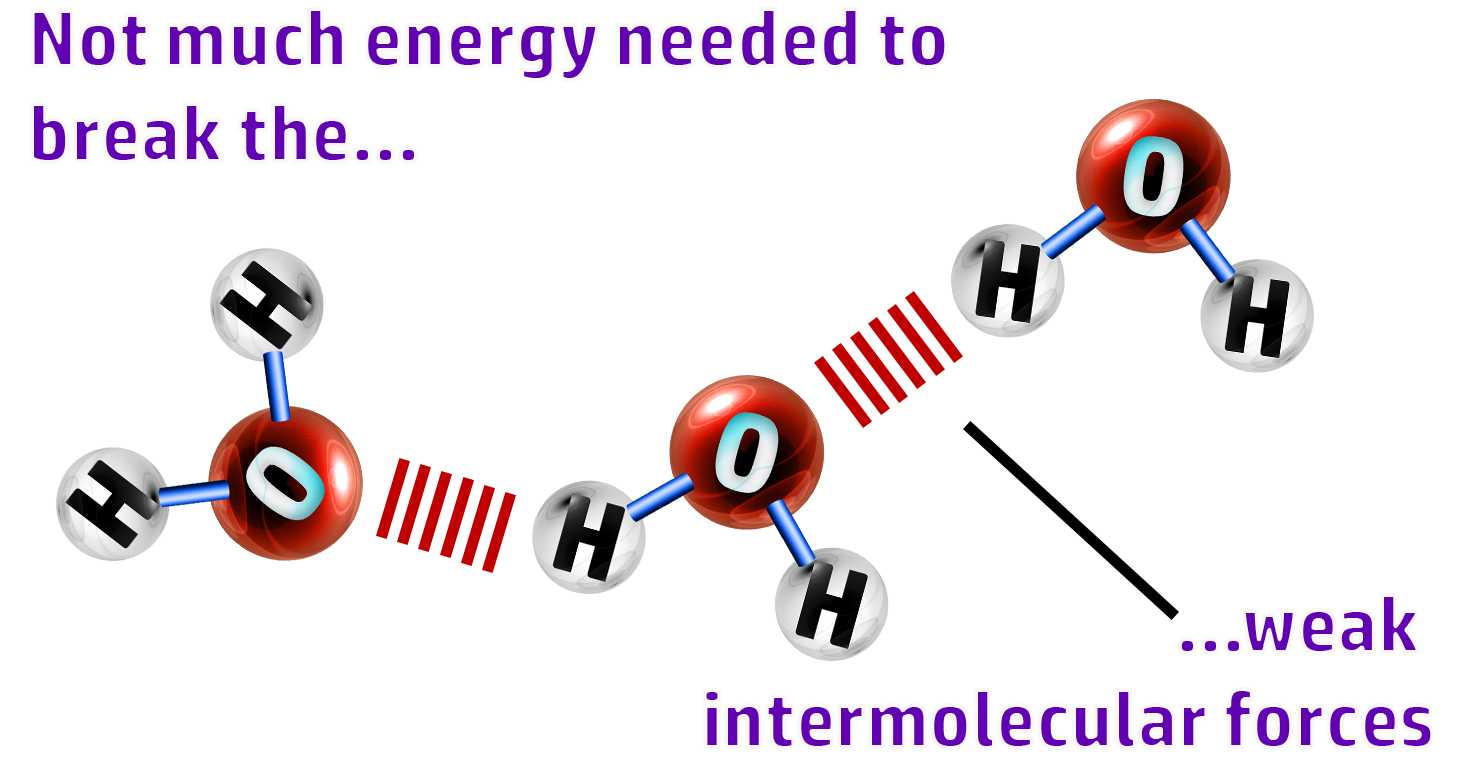
CH62: Explain why simple molecular substances have low melting points
They have low melting point and boiling points
Not much energy is needed to break the weak intermolecular forces (forces between the molecules)
CH63: Explain why simple covalent substances do not conduct electricity
They cannot conduct electricity.
The electrons are not delocalised (free), so cannot move/flow.
This means they cannot carry a charge.

CH64: Describe what a giant covalent substance is
Giant covalent substances are bonded the same way as seen in simple covalent molecules. They still have shared pairs of electrons and involve only non-metals.
The only difference is the number of atoms and bonds involved – there are a lot more.
This gives giant covalent substances a high melting point because lots of energy is needed to break the strong covalent bonds.
They also normally don’t conduct electricity (there are some exceptions, such as graphite, graphene and C60) because the electrons are not delocalised (free), so cannot move/flow.
Diamond and Graphite are both examples of allotropes of carbon – a substance made up of the same element but with a different chemical structure
CH65: Describe the structure of diamond and graphite
Diamond
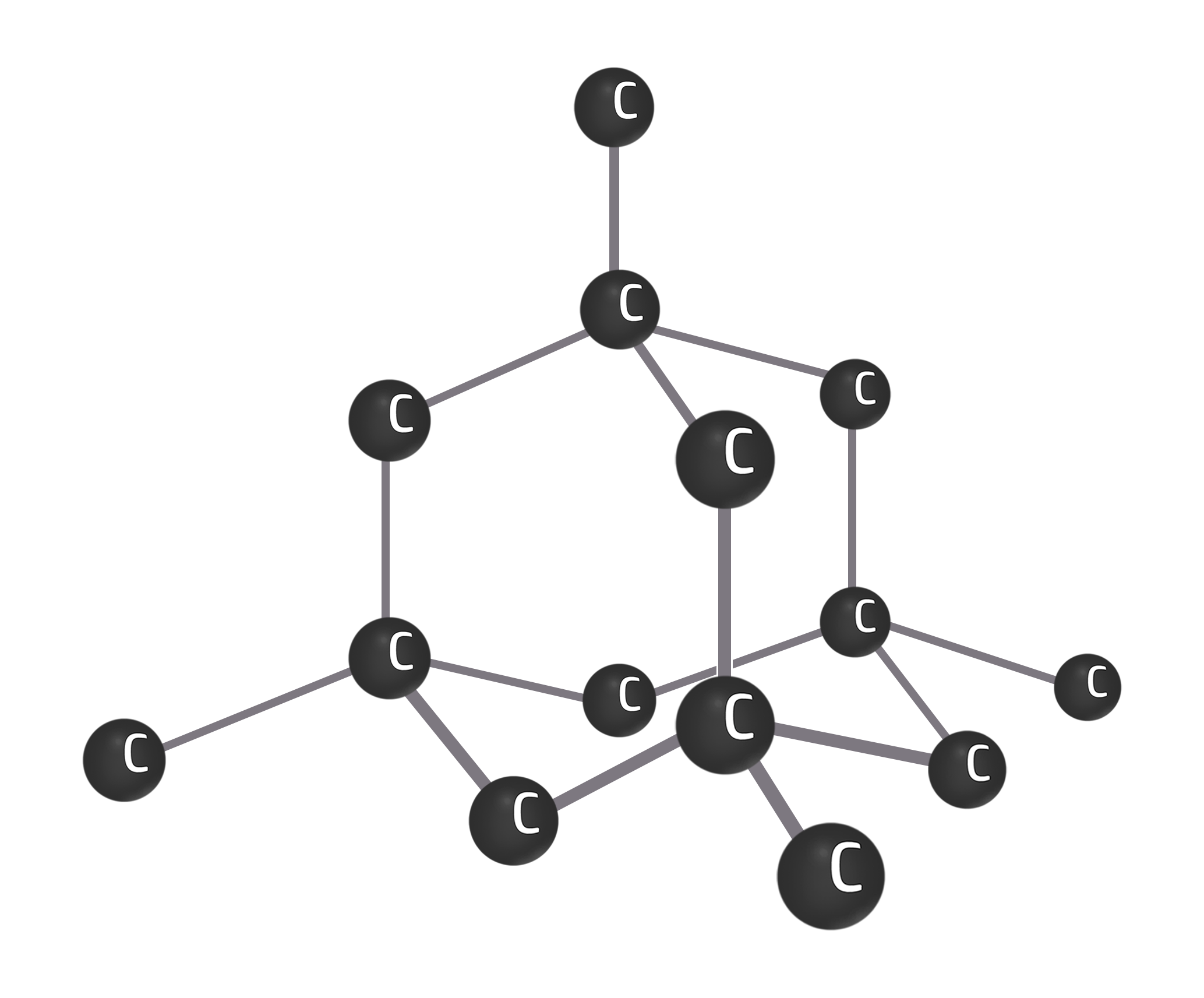
Diamond is an allotrope of carbon (same element, different structure) where every carbon has 4 strong covalent bonds.
Graphite
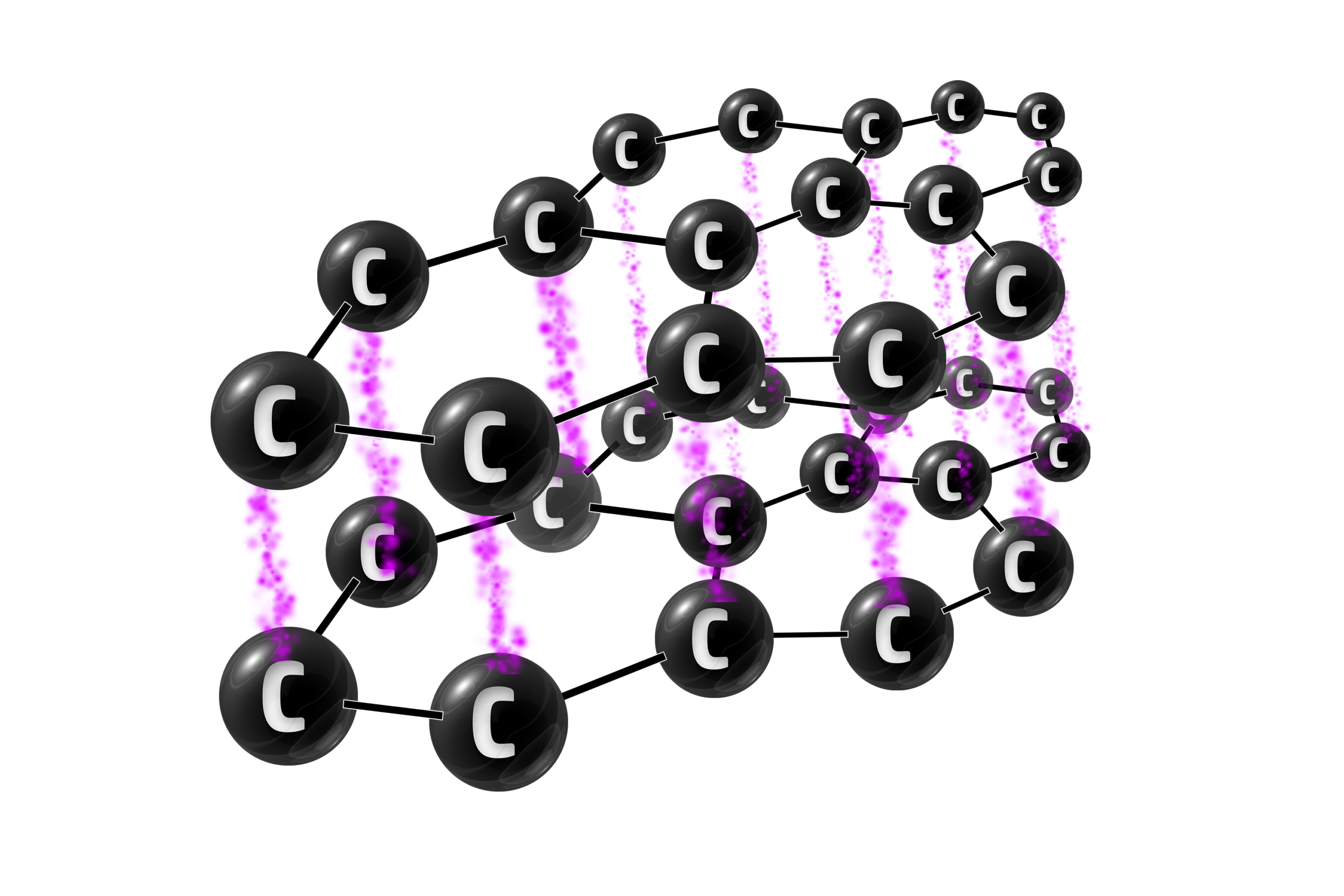
Graphite is an allotrope of carbon (same element, different structure) where every carbon has 3 strong covalent bonds. This gives it thin layers of carbon atoms.
CH66: Explain the uses of diamond and graphite
Diamond
Diamond has 4 strong covalent bonds between each carbon atom. This makes diamond strong.
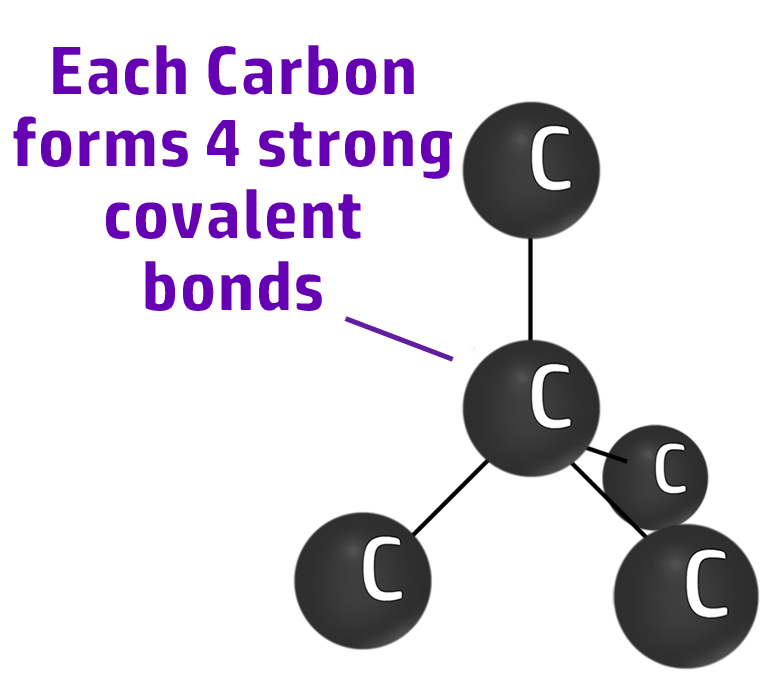
Diamond can be used in cutting tools because it takes a lot of energy to break the strong covalent bonds.
Graphite
Graphite has 3 strong covalent bonds between each carbon atom.
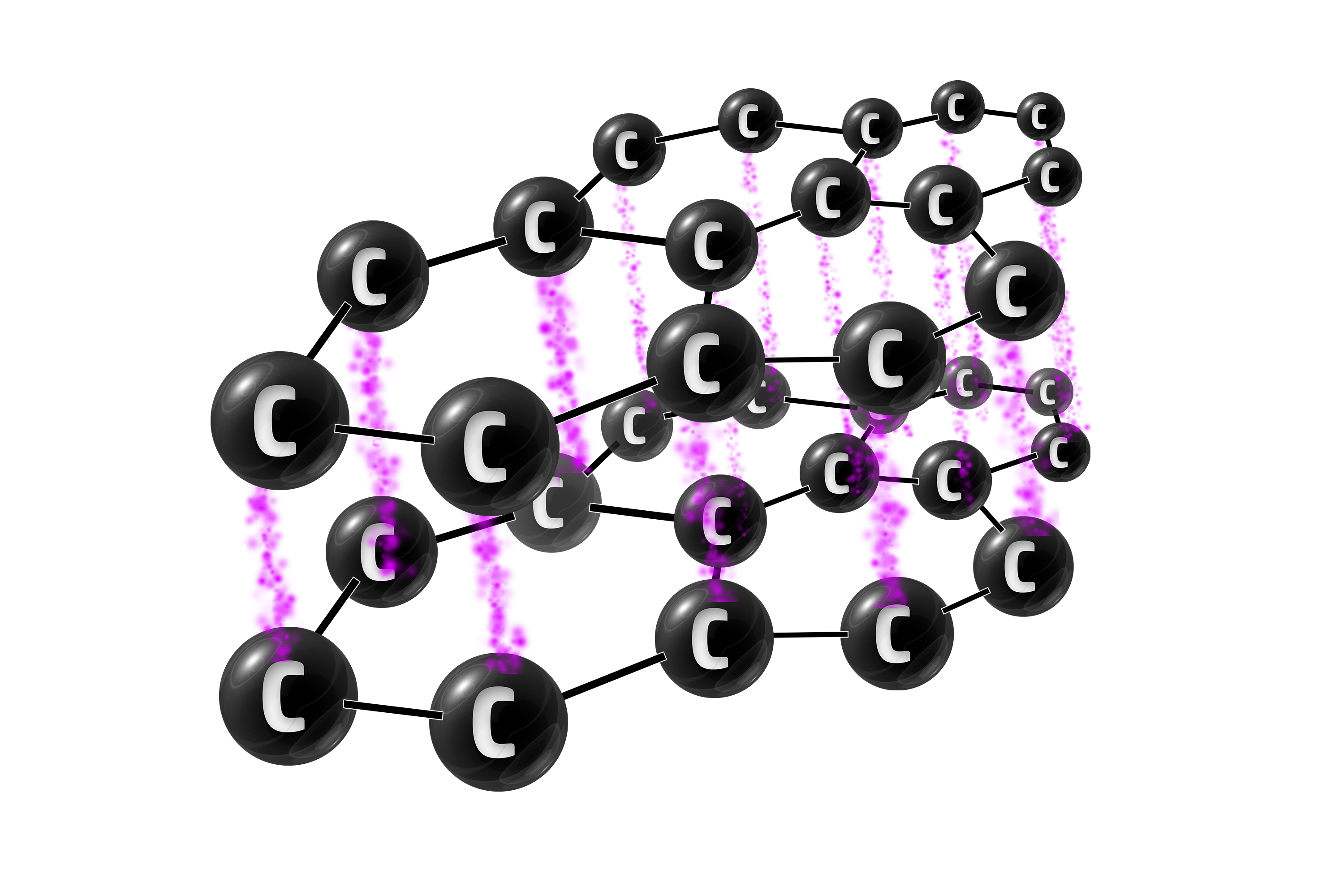
Graphite can be used in electrodes because the delocalised electrons are free to move – allowing it to conduct electricity.
Graphite is also used as a lubricant in cars because it has layers which slide past each other – reducing friction.
CH67: Describe the properties of graphene, fullerenes and nanotubes
Graphene
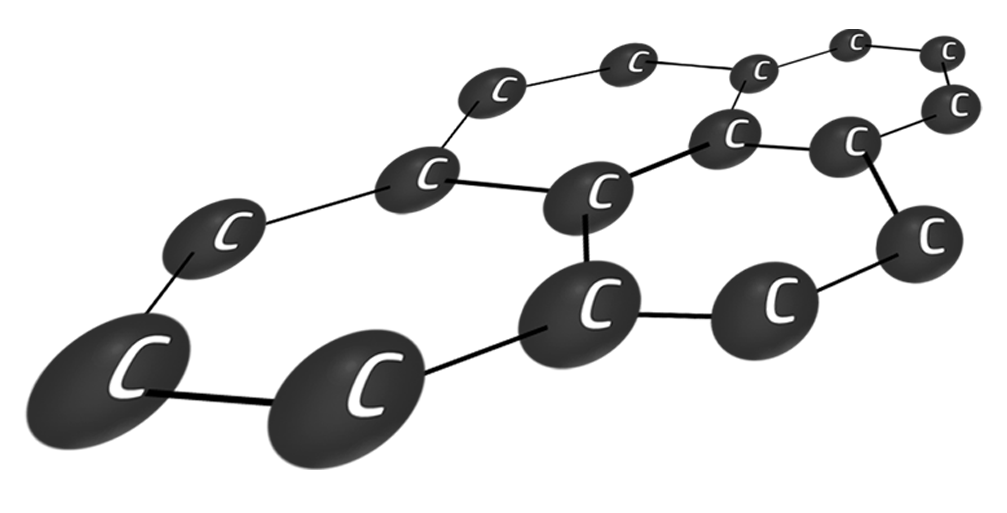
Graphene is one layer of graphite.
It is light (1 atom thick) and strong (lots of strong covalent bonds). It also conducts electricity because the delocalised electrons are free to move.
Buckyballs
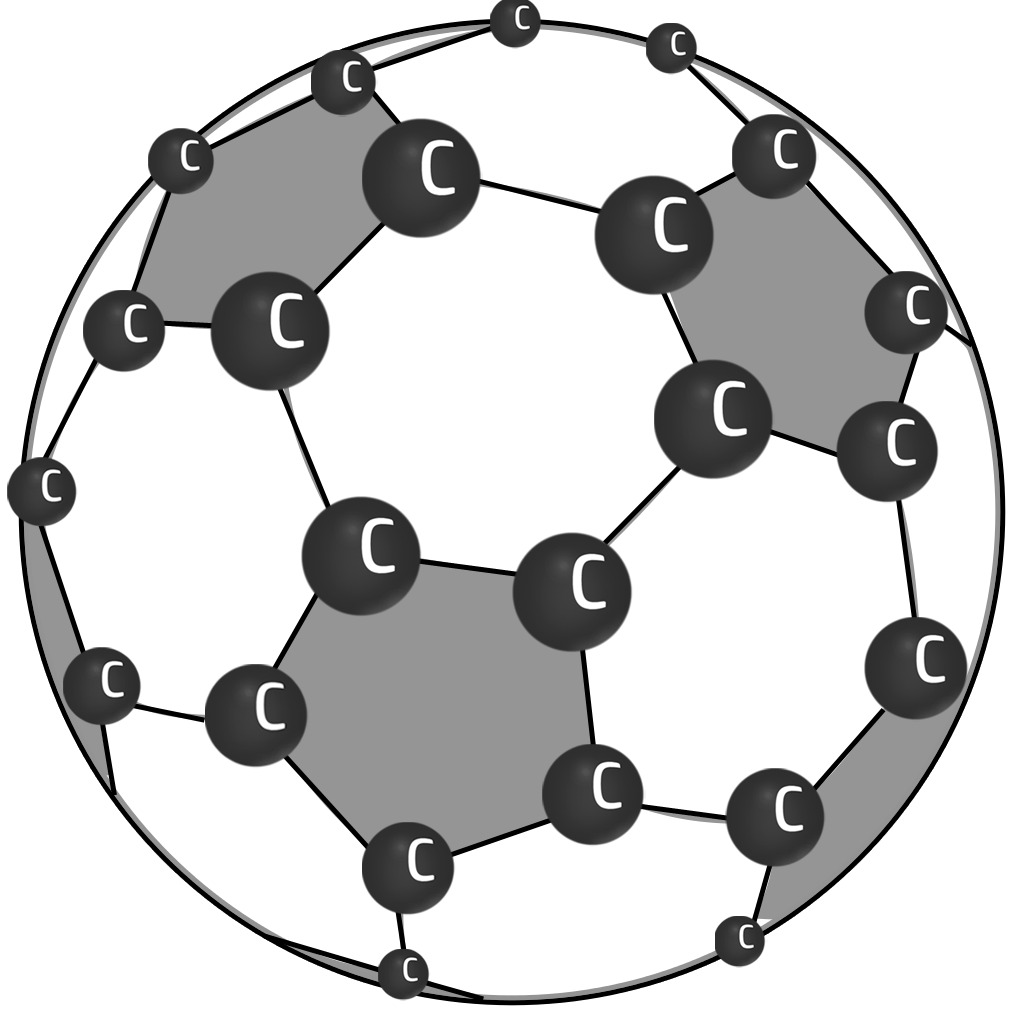
Simple covalent spheres of carbon atoms.
They conduct electricity because the delocalised electrons are free to move. They have low melting points because not much energy is needed to break the weak intermolecular forces.
Nanotubes
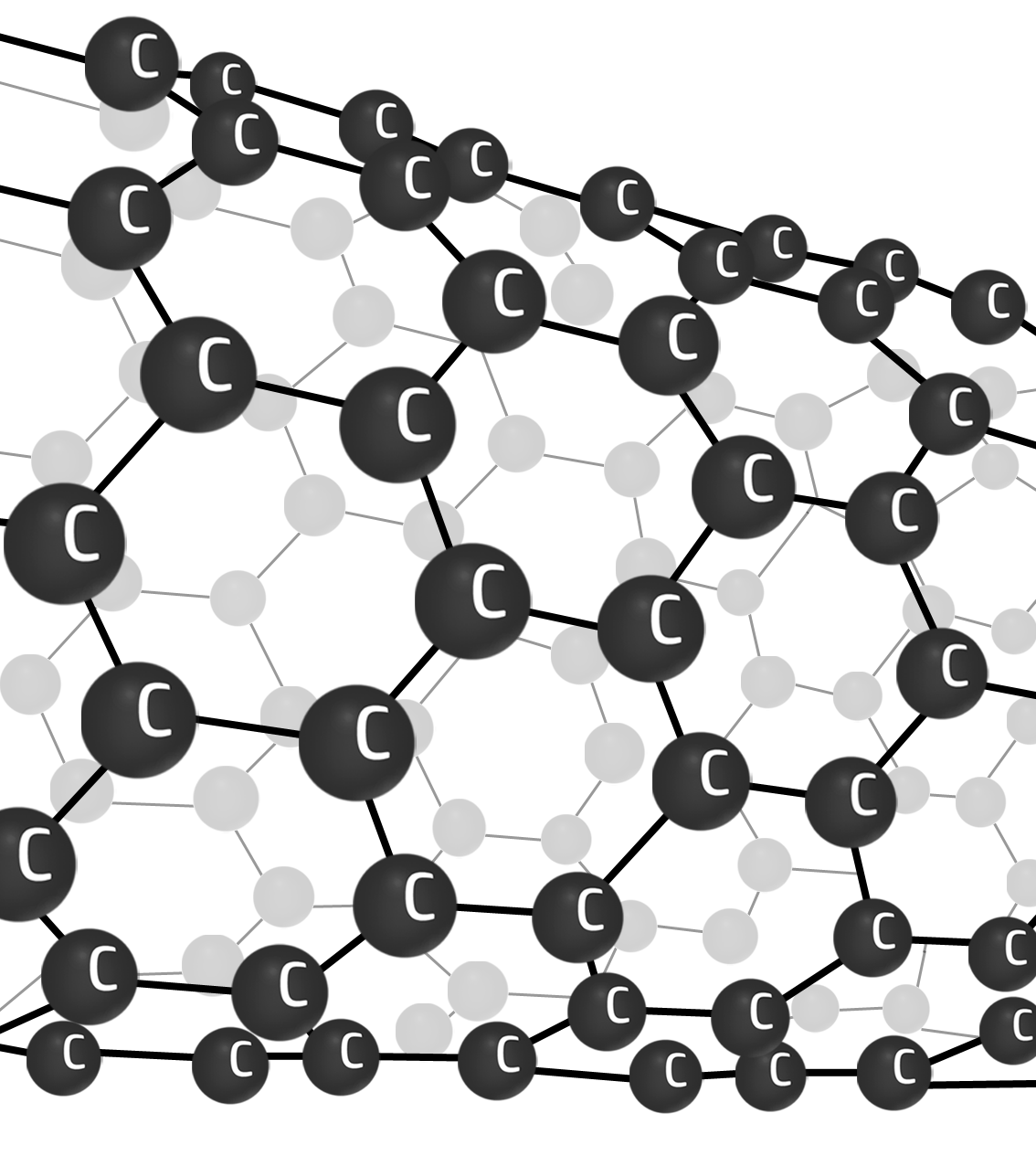
A ‘rolled up’ sheet of graphene.
Nanotubes have high tensile strength (can’t be stretched). They are strong (lots of strong covalent bonds) and can conduct electricity (delocalised electrons flow).
