Atomic Structure and the Periodic Table
A GCSE chemistry revision page on atomic structure covering subatomic particles, electronic structure and shells, isotopes, relative mass/calculations, how to draw atom diagrams, and the development of atomic models with examples and explanations.
CH27: Describe how ideas of the atom have changed
1. Dalton described atoms as indestructible spheres that could not be broken down.
2. Dalton suggested that atoms of an element were all identical.
3. JJ Thompson measured the mass of rays in a cathode tube. They were lighter than atoms – proving that the atom was not indestructible.
4. JJ Thompson proved that electrons existed – the plum pudding model – negative electrons = the plums, positive atom = the pudding.
5. Rutherford fired 20,000 alpha particles into gold foil. Most went through, some refracted and a few repelled. This proved the nucleus was positive and tiny.
6. Today’s atom, proposed by Niels Bohr, is known to contain protons and neutrons in the nucleus, with electrons orbiting shells.
CH28: Describe the structure of the atom.
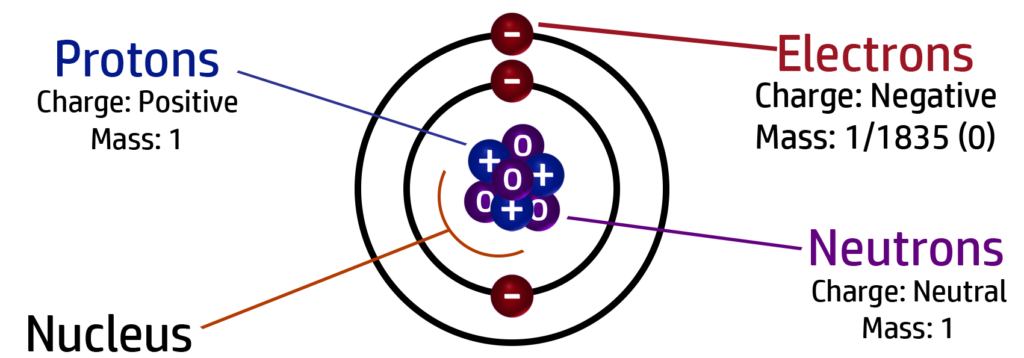
CH29: Identify the charge and mass of a proton, neutron and electron
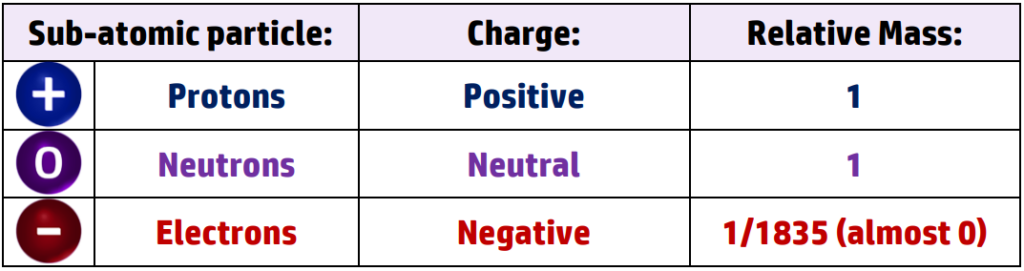
CH30: Explain why all atoms are neutral.
All atoms are neutral because they have the same number of protons and electrons. The charges cancel each other out giving an overall charge of 0.
For example: Lithium has 3 protons, 4 neutrons and 3 electrons:
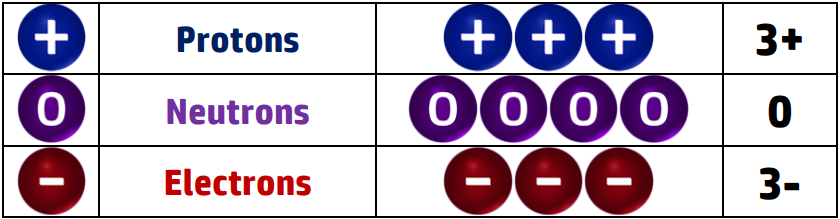
There are 3 x positive protons and 3 x negative electrons - +3-3 = 0 which is the overall charge. The neutrons don’t impact on the charge because they are neutral.
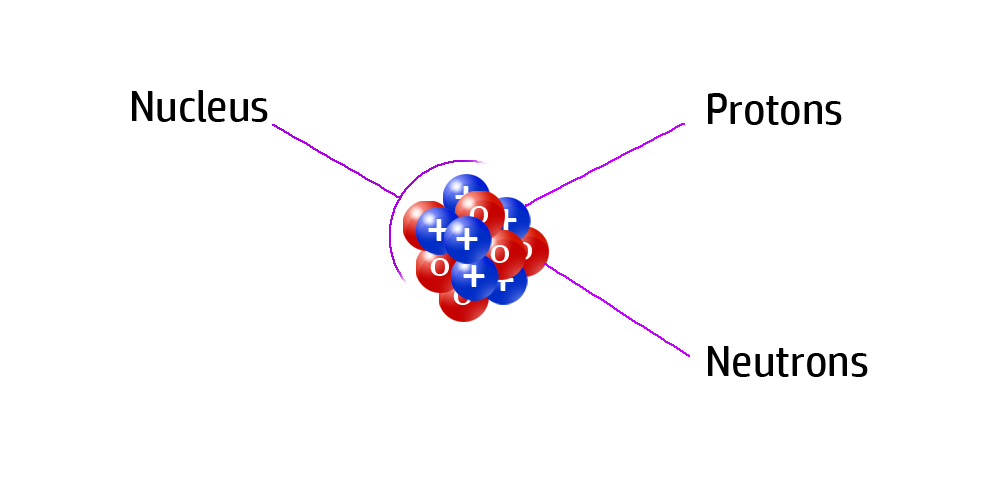
CH31: Compare the size of the atom to the nucleus.
The nucleus is tiny.
Imagine a football field – this is the size of your atom.
Now imagine a marble on that football field. The marble is the size of the nucleus compared to the rest of the atom!

CH32: Identify where most of the mass of the atom is.
Most of the mass of an atom is found in the nucleus.
This is because the nucleus contains protons and neutrons, which both have a relative mass of 1.
Electrons have a relative mass of almost 0 (1/1835) so don’t add much mass to the atom.
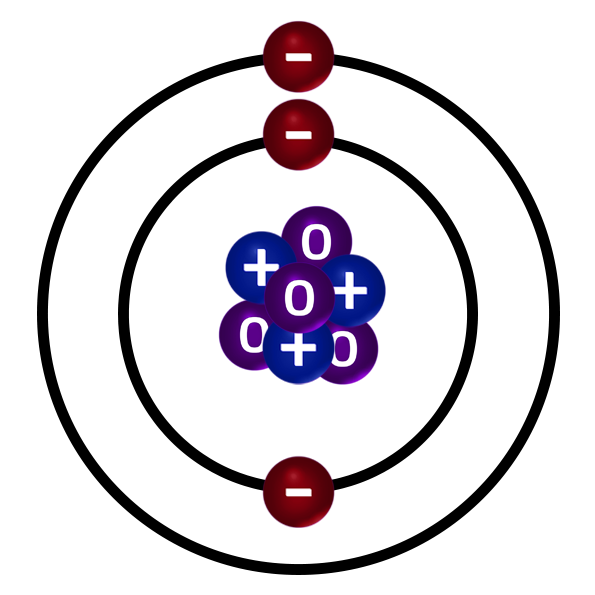
CH33: What does the mass number tell us?
The mass number is the total number of protons and neutrons inside the nucleus of an atom.
It is usually the largest number for each atom on the Periodic Table.
For example, the atom on the right has 3 positive protons and 4 neutral neutrons in the nucleus of its atom. Therefore, the mass number of this atom is 7.
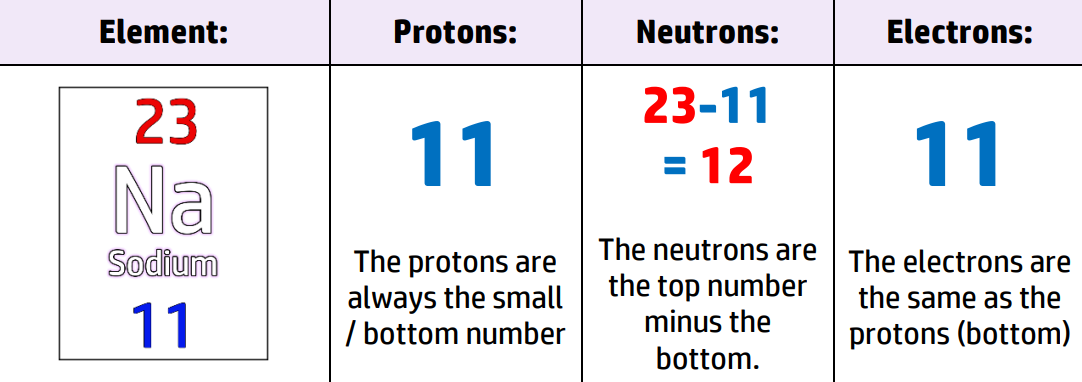
CH34: Describe why the atomic number is unique.
The atomic number is the number of protons in an atom. It is also the smaller/bottom number in the Periodic Table.
This does not change (unlike neutrons and electrons). Therefore, if you have 11 protons, you always have an atom of Sodium, Na.

CH36: Describe how to draw the electronic configuration.

Find out the number of electrons. Aluminium has 13.
The first shell can hold 2e- and the 2nd/3rd can hold 8e-.
Fill up the shells from the inside out.
Aluminium can hold 2e- on the first shell, 8e- in the second and has 3e- left for the third shell.

CH37: Relate the electronic configuration to the periodic table
The number of electrons on the outer shell tells you the group the element is in.
Chlorine is in group 7 because it has 7 electrons in the outer shell.
The number of shells tells you the period the element is in.
Chlorine is in period 3 because it has 3 shells.

2.8.7
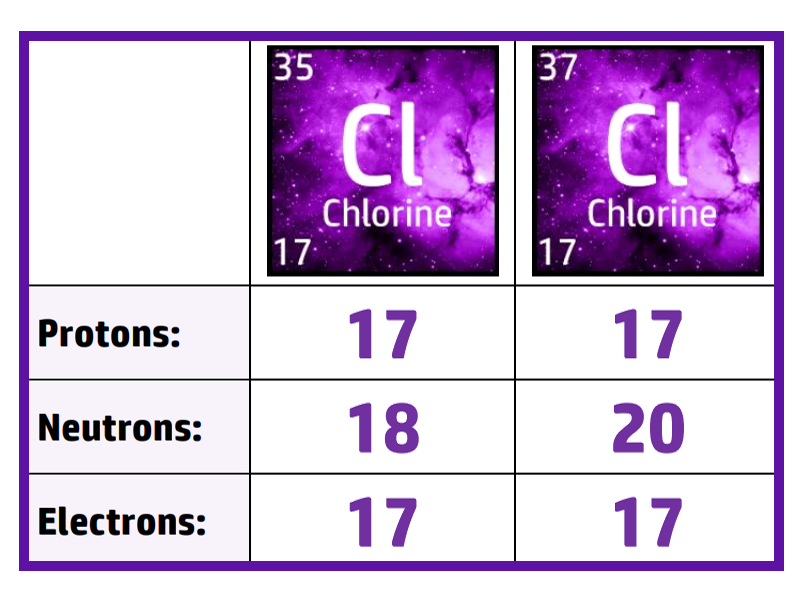
CH38: Describe what an isotope is
An isotope is an atom that has the same number of protons, but different number of neutrons.
In the example of chlorine on the right – both isotopes have 17 protons (same), but chlorine-35 has 18 neutrons, whilst chlorine-37 has 20 neutrons (different)
We don’t include electrons in the definition because they can change when forming ions.
CH39: Explain why not all mass numbers are whole numbers
On some Periodic Tables you will see that elements don’t all have whole numbers (such as chlorine having a relative mass of 35.5 and boron having a relative mass of 10.8)
This is because the mass is the average mass of all of the isotopes. Sometimes this average is not a whole number.

CH40: Calculate the relative atomic mass of an isotope (H)
To calculate the relative atomic mass of an isotope you need to follow the following steps:

For example, if Chlorine has two isotopes – 35-Cl, with an abundance of 75% and 37-Cl with an abundance of 25%, the calculation will look like this:
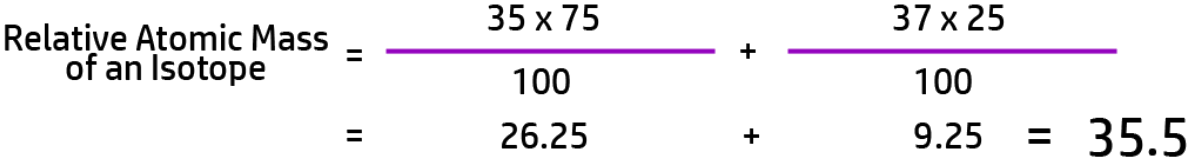
CH41: Estimate the abundance of isotopes (H)
Example: There are two isotopes of copper. 63-Cu and 65-Cu. The relative atomic mass is 63.5. Estimate the abundance of each isotope.
- Step 1: Work out the difference between the masses of each isotope. 65-63= 2.
- Step 2: Work out the difference between the isotope mass and each isotope:
- 63.5-63 = 0.5
- 65-63.5 = 1.5
- Step 3: Work out the percentage for each isotope by dividing these differences (step 2) by the total difference (step 1)
- 0.5 / 2 x 100 = 25%
- 1.5 / 2 x 100 = 75%
- Step 4: The isotope with the mass closest to the relative atomic mass is the largest abundance. So, 63-Cu is 75% abundant and 65-Cu is 25% abundant.
CH42: Describe how Mendeleev arranged his Periodic Table
Dmitri Mendeleev was a Russian Scientist who, in 1869, decided to put the elements know at the time into a Table of Elements.
He put the elements into groups based on their chemical properties – which is still the same today. He also arranged the elements in order of atomic mass. We now know that this was wrong and today’s Periodic Table is in order of atomic number.

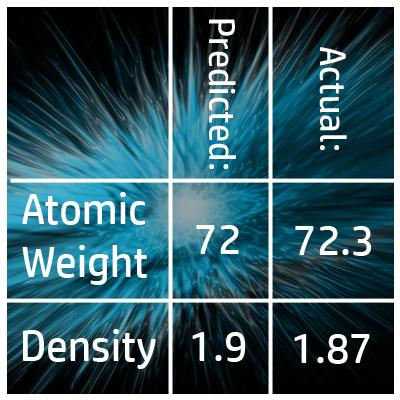
CH43: Explain how Mendeleev predicted the properties of undiscovered elements
If an element hadn’t been discovered yet, Mendeleev left gaps and used the properties from the elements in the rest of the group to predict the properties of the missing elements.
For example, he predicted the atomic weight of Germanium was 72 and the density 1.9g/dm3. Many years later, when these elements were discovered, he was almost spot on!
CH44: Explain what Mendeleev did when properties didn't match
Because Dmitri Mendeleev’s table of the Elements was in order of atomic mass (but should have been in order of atomic number), some of the properties didn’t match up to the properties of the group.
He ended up swapping these elements so that the chemical properties matched. An example of this is iodine (group 7) and tellurium (group 6). Originally, iodine was in group 6 and tellurium was in group 7, but iodine has similar chemical properties to the rest of the halogens (group 7) – so Mendeleev swapped them.
CH45: Describe how the atomic number is linked to the Periodic Table
The atomic number tells you where the atom can be found on the Periodic Table.
Starting off with Hydrogen (Period 1), the atomic number increases by one each time you go from left to right.
So, if you have an atomic number of 3 (Lithium) and move 5 places to the right, you will have an atomic number of 8 (oxygen)
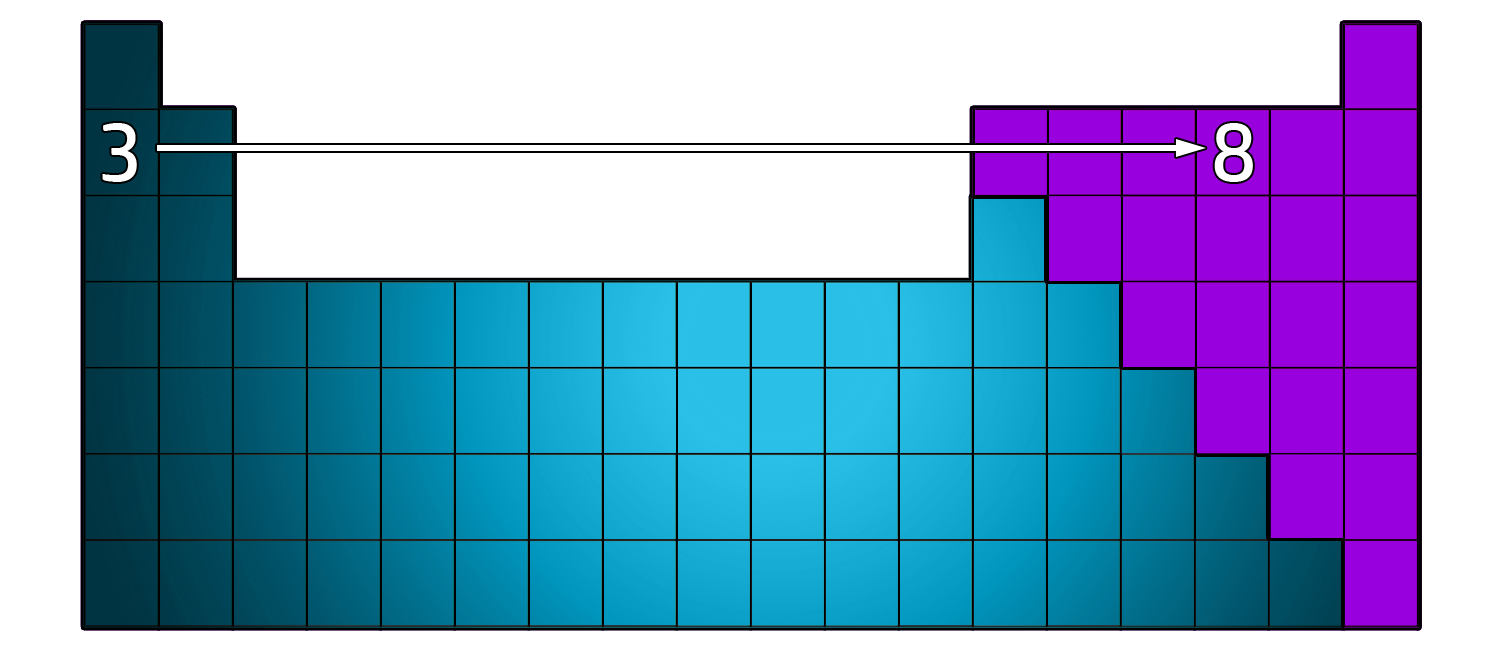
CH46: Describe the structure of the Periodic Table
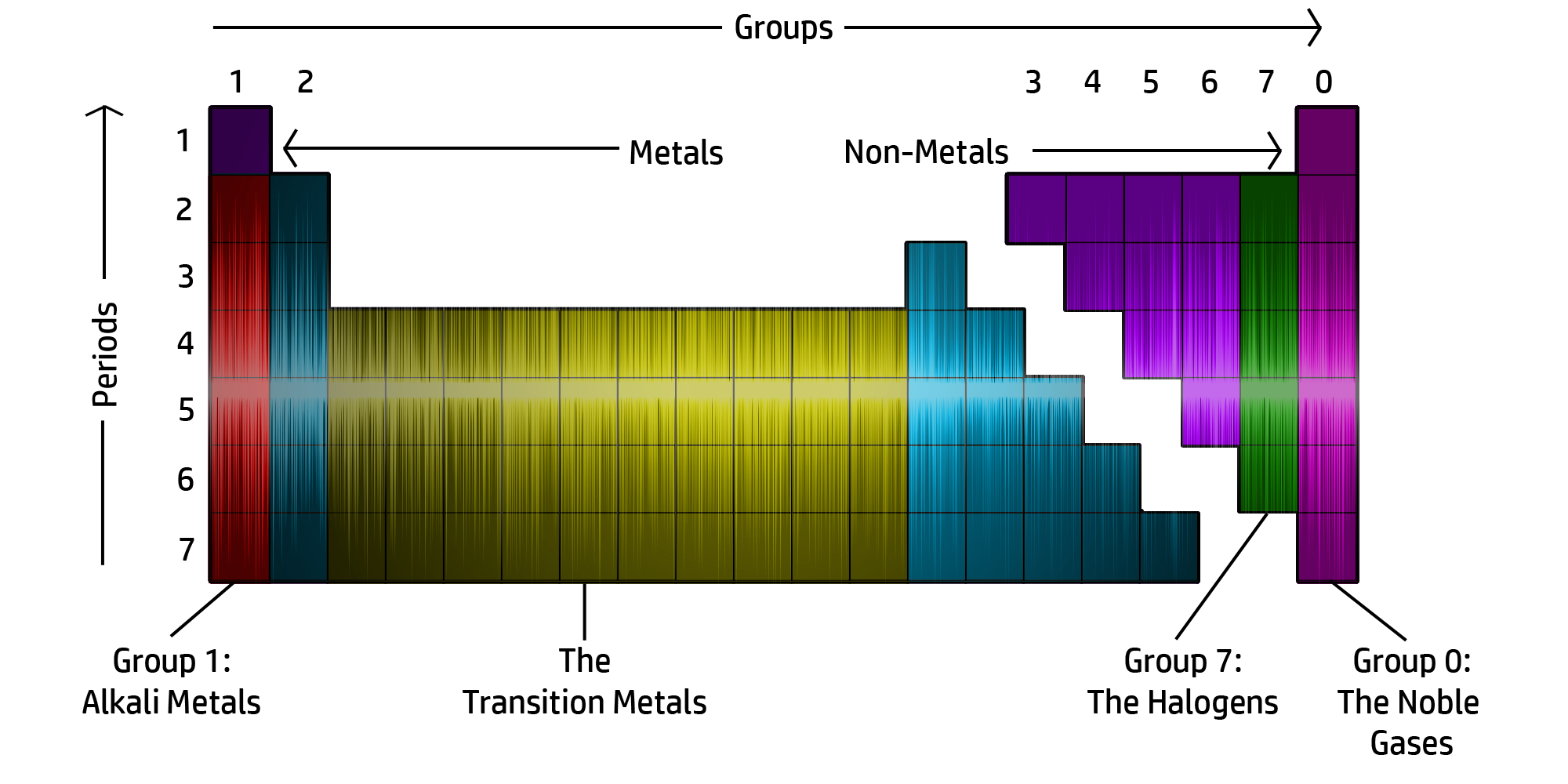
The Periodic table is now arranged in groups based on chemical properties.
The groups can be seen in the diagram above.
CH47: Identify where metals / non-metals are found on the Periodic Table
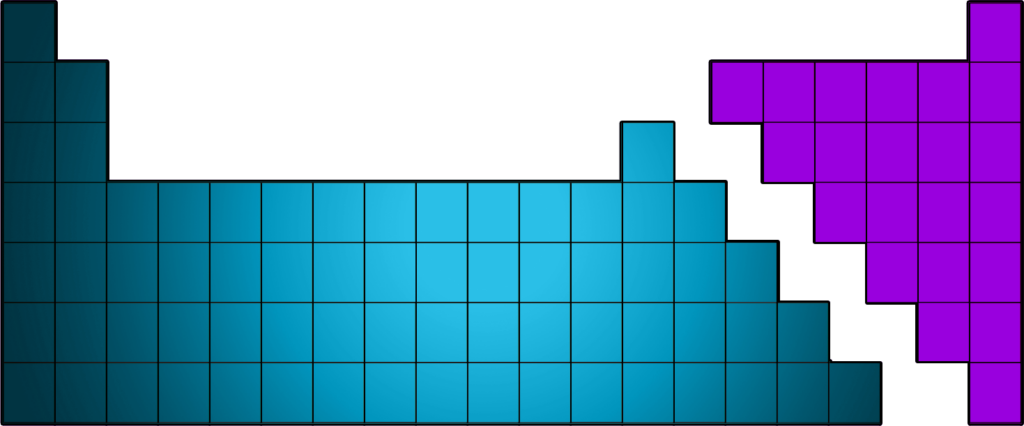
- Metals are found on the left of the zigzag (with the exception of hydrogen, which is a non-metal.
- Non-Metals are found on the right of the zigzag.
The zigzag starts between Boron and Aluminium and goes right and down like a staircase!