Types of Substance
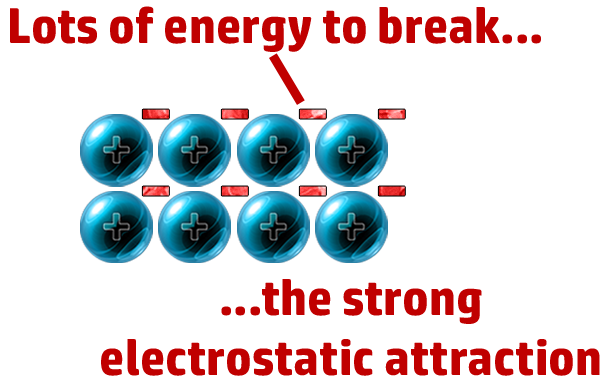
CH68: Explain why metals have high melting points
Lots of energy needed to break the strong electrostatic attraction between cation and delocalised electrons.
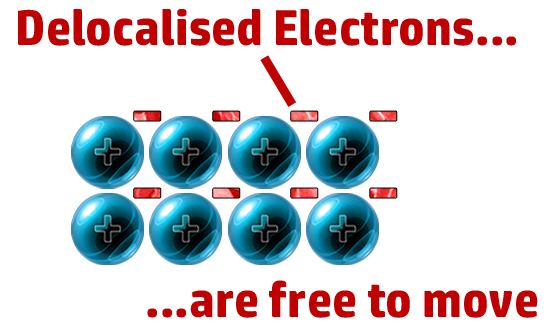
CH69: Explain why metals conduct electricity
Delocalised electrons can flow/move and carry a charge
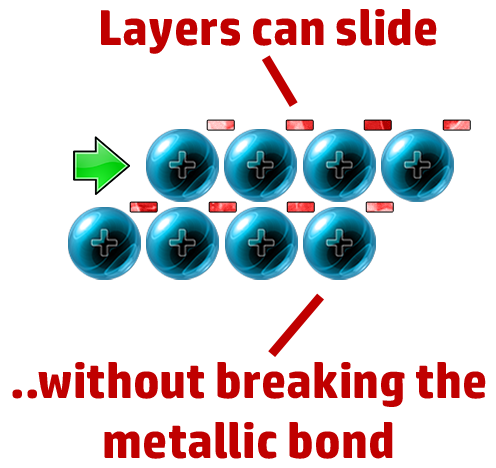
CH70: Explain why metals are malleable
The layers can slide past each other, but it doesn’t break the strong electrostatic attraction between cation and delocalised electron.
CH71: Identify the properties of metals and non-metals
| Properties of Metals: | Properties of non-metals: |
| Good conductors of heat & electricity | Poor conductors of heat & electricity |
| Strong | Brittle (Break easily) |
| Malleable (Can be hammered into shape) | Low Melting Points & Boiling Points |
| Ductile (Can be stretched into wires) | Low Density |
| High Melting Points & Boiling Points |
CH72: Explain what a polymer is
A polymer is a long chain of smaller molecules called monomers.
These are examples of simple covalent compounds and have low melting points and cannot conduct electricity.
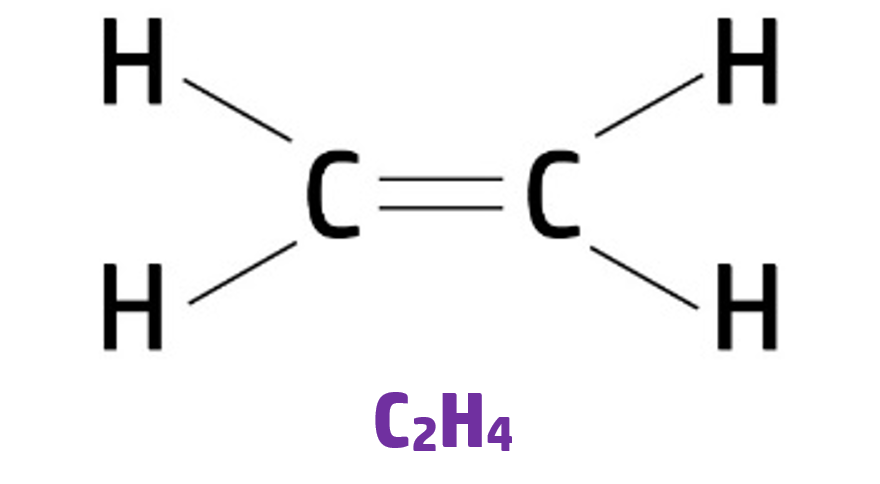
An example of this is ethene – which has the formula C2H4:
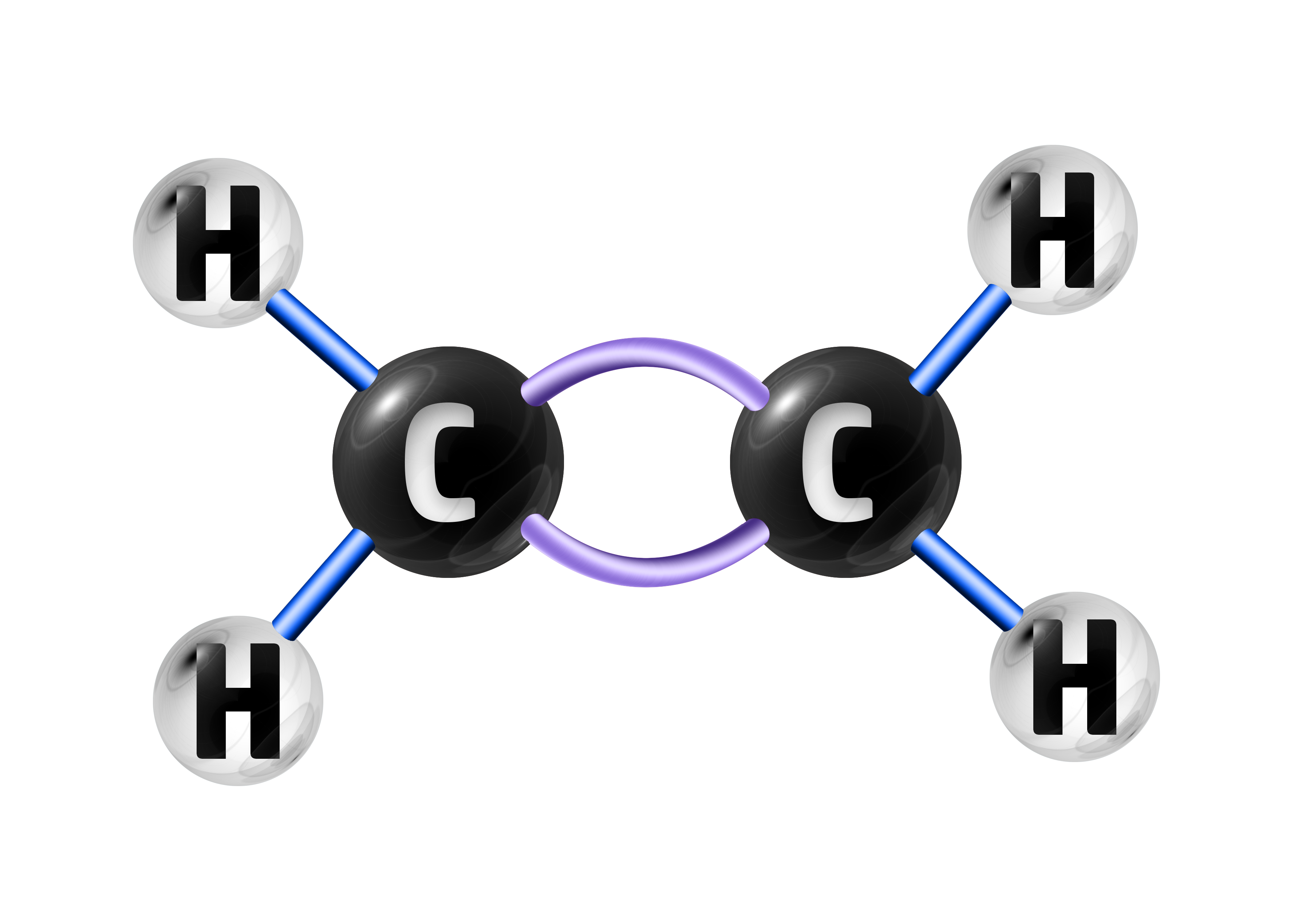
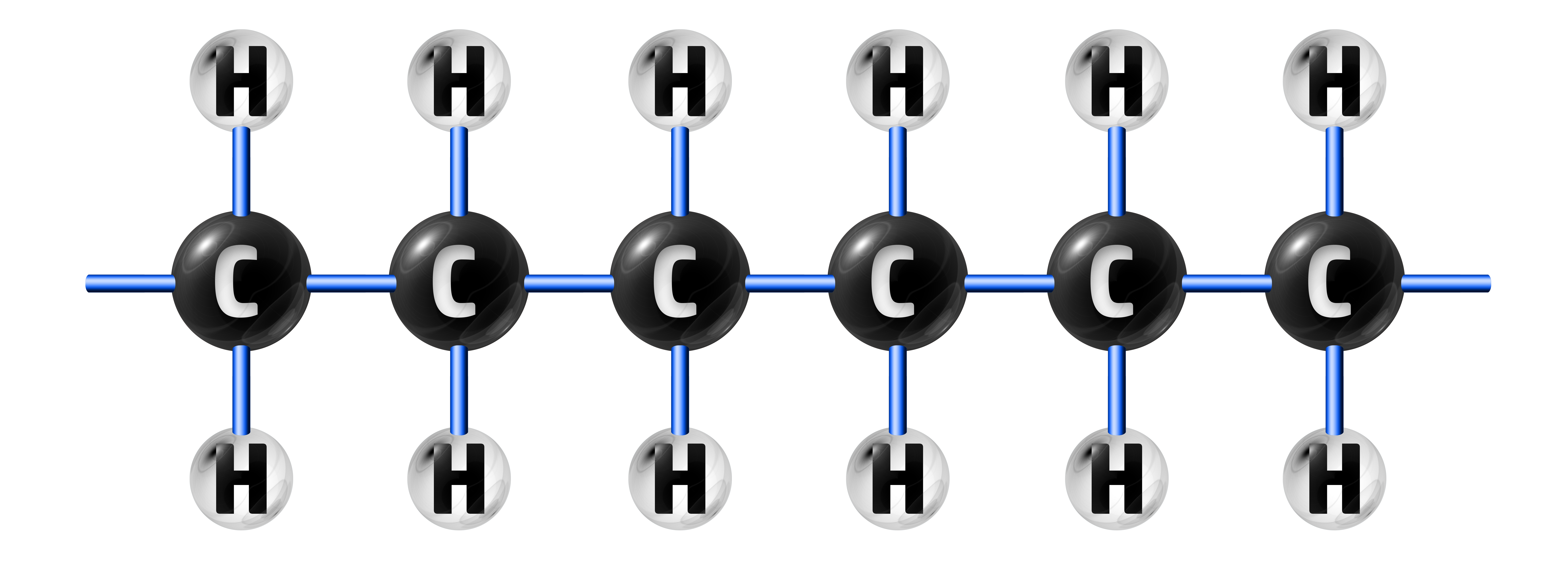
When the double bond is broken, many ethene molecules will join together to make poly(ethene):
CH73: Evaluate the different types of models
2D Models:
Advantages:
- Simple.
- Shows the atoms involved.
- Shows how the atoms are connected.
Disadvantages:
- Doesn’t show the shape of the substance.
- Doesn’t show the size of the substance.
Dot and Cross:
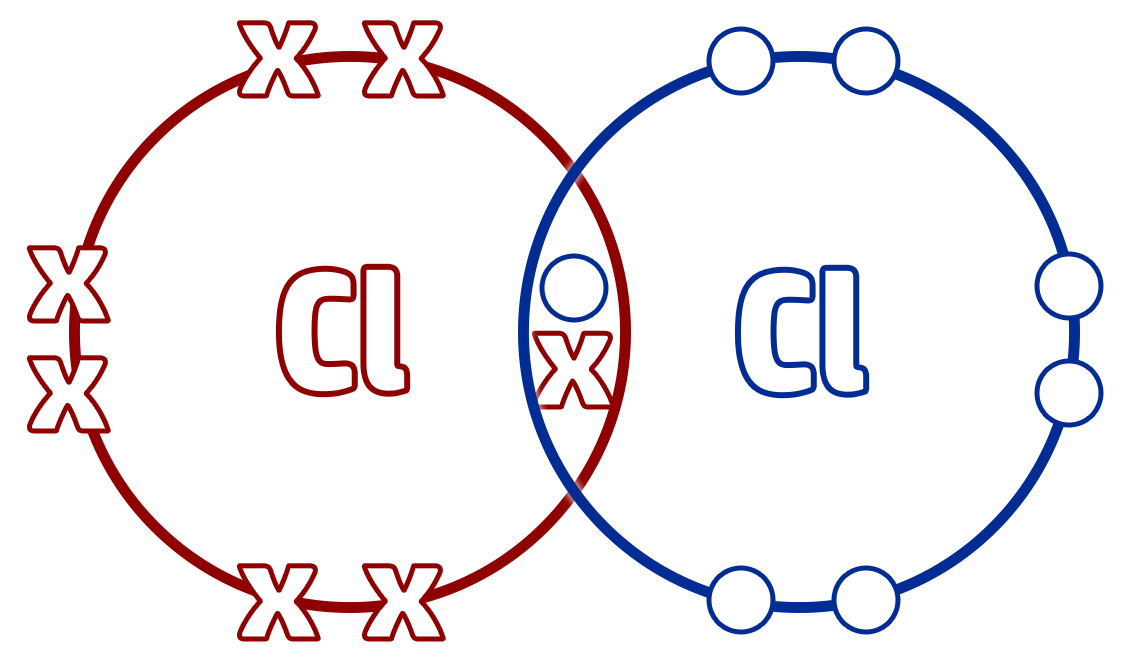
Advantages:
- Shows the electron arrangement.
- Shows how substances are formed.
- Shows where the electrons are from.
Disadvantages:
- Doesn’t show the size of the substance.
- Doesn’t show you how the ions are arranged.
3D Models:
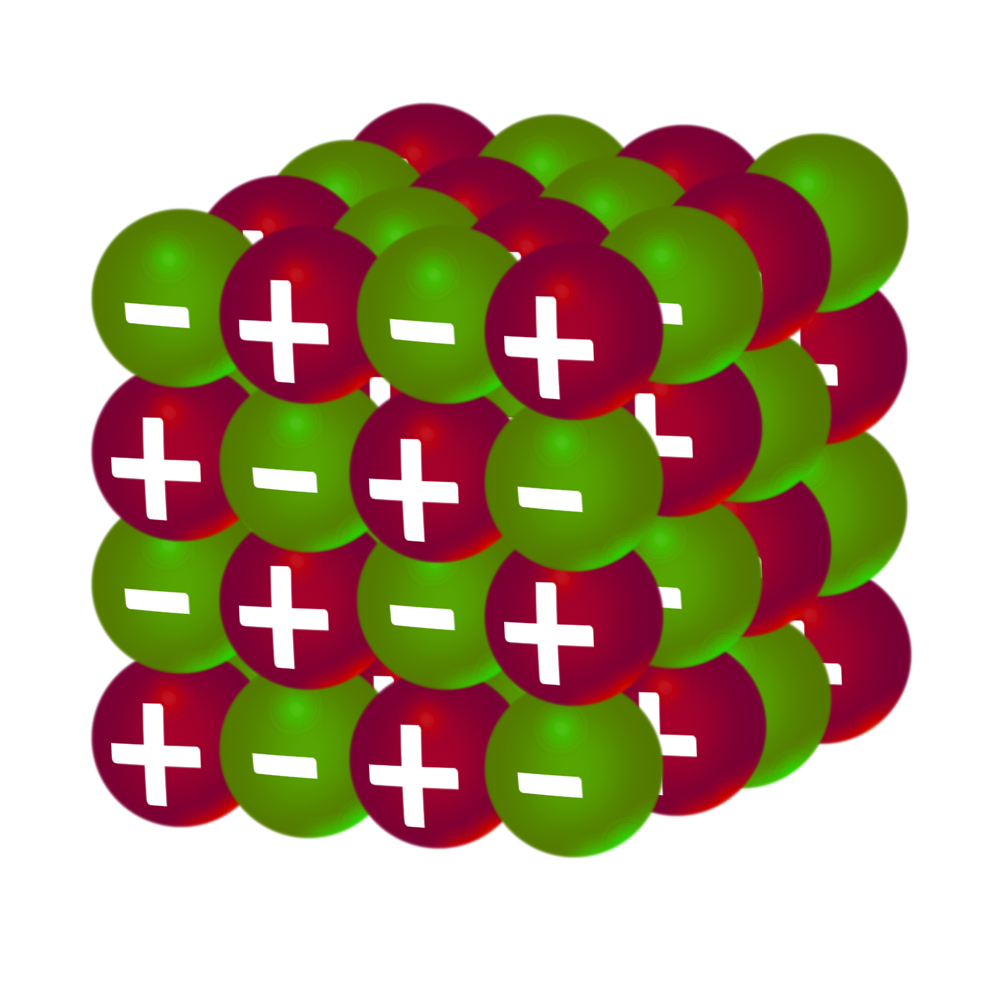
Advantages:
- Shows the arrangement of the ions.
- Shows the shape of the substance
Disadvantages:
- Only shows the outer layer of the substance.
- Doesn’t show the correct size of the atoms.
Ball and Stick:
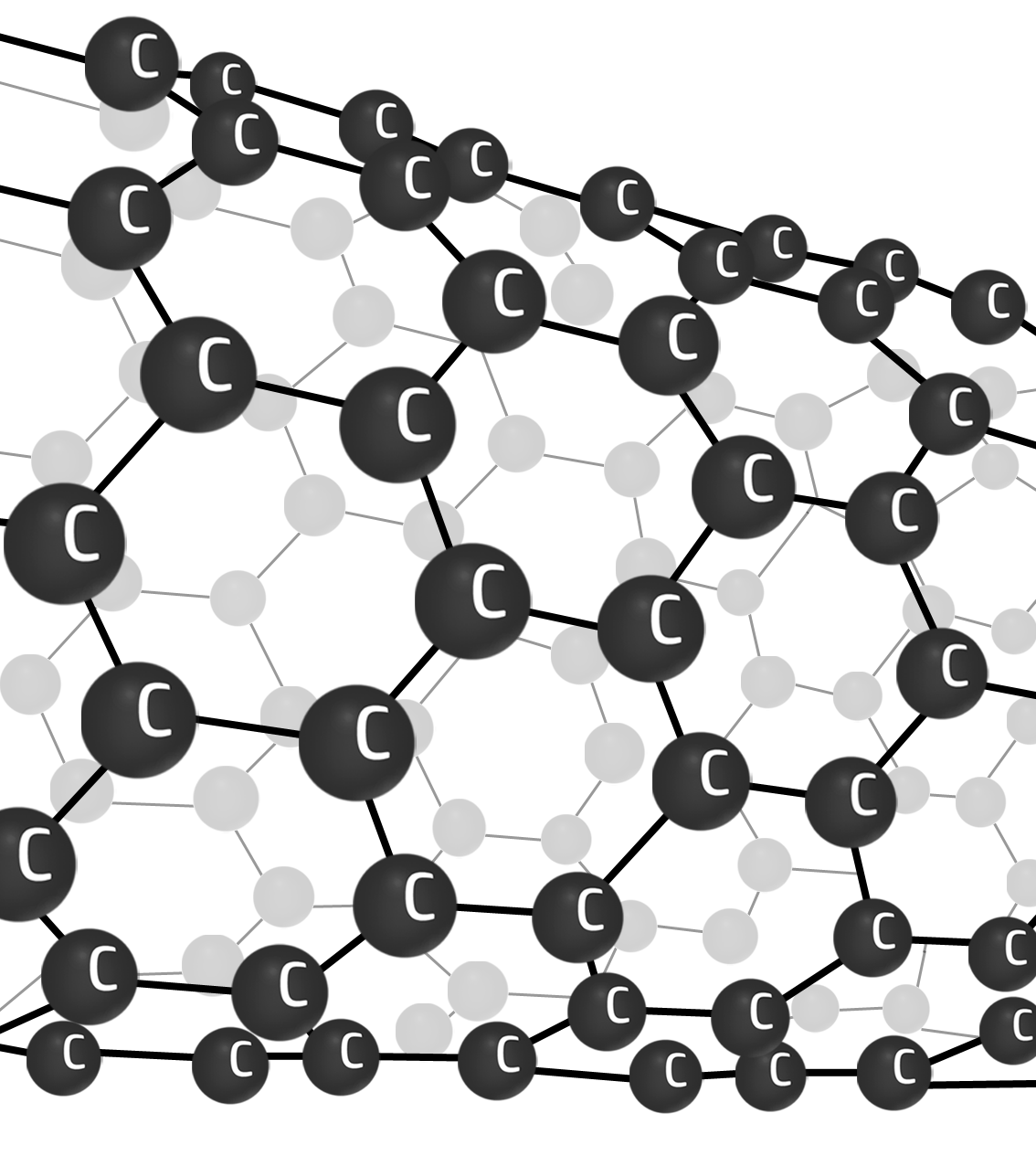
Advantages:
- Shows the shape of the lattice/structure in 3D.
- Show the atoms and their arrangement.
- More realistic than 2D drawings.
Disadvantages:
- Misleading – the gaps shown are not really there – that’s where the electrons are.
- Doesn’t show the correct size of the atoms.
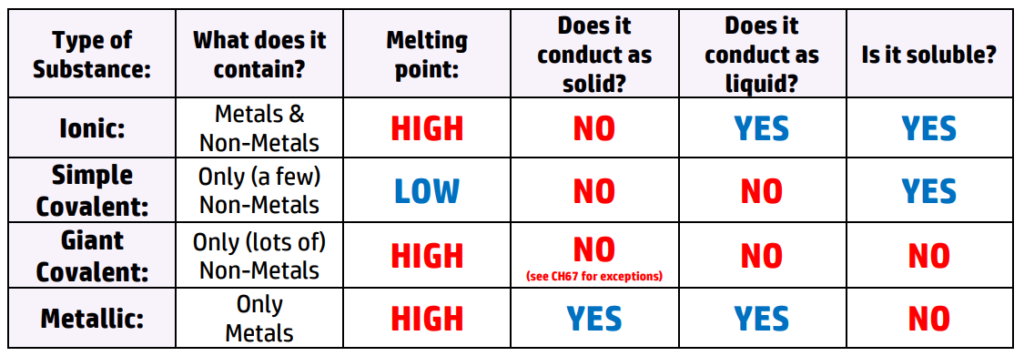
CH74: Identify the properties of ionic, covalent and metallic substances
CH75: Analyse data to identify the type of bonding occurring
Example: Look at the table below – can you name the type of bonding for each?
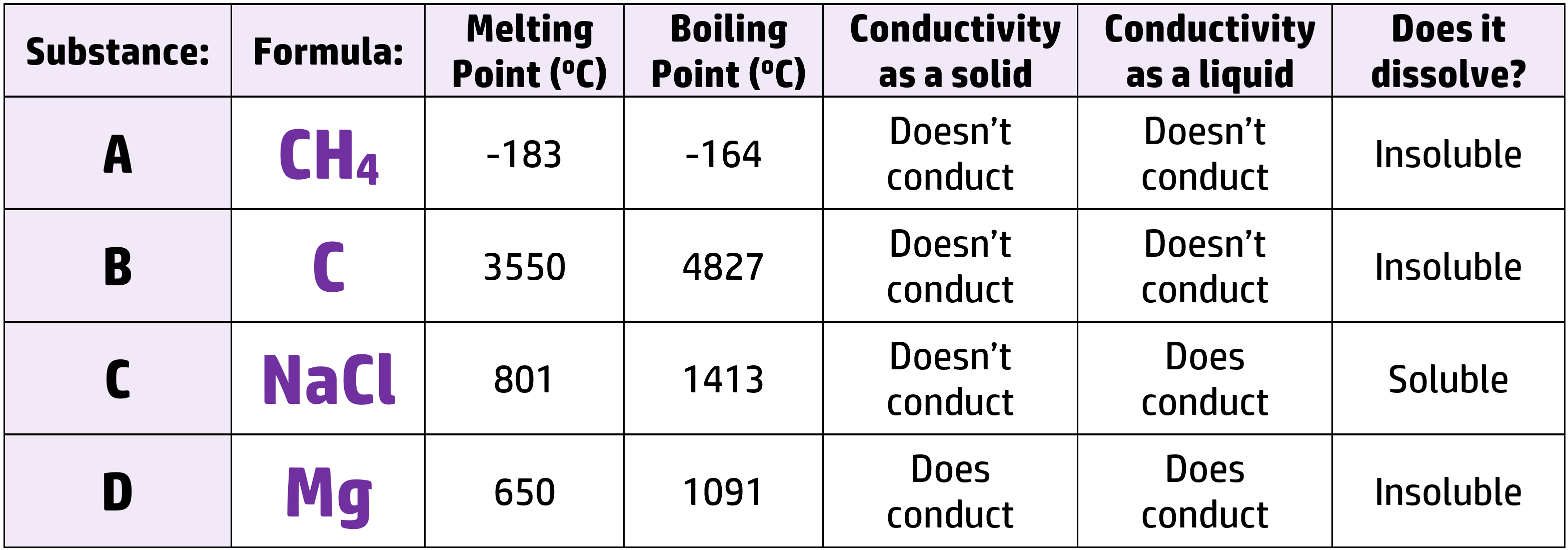
- Substance A is simple covalent because it has a low boiling point. (It is the only type of bonding with a low boiling point!!)
- Substance B is giant covalent because it has a high melting point (ionic, giant covalent and metallic), is made of only non-metals, and doesn’t conduct electricity.
- Substance C is ionic because it conducts electricity when liquid, but not when solid. (It is the only type of substance that only conducts when liquid/molten/dissolved).
- Substance D is metallic because only metals conduct electricity when both liquid and solid.
CH76: Investigate the type of bonding occurring
There are lots of ways to find out if a substance is ionic, covalent or metallic. Here are a few:
- Heat the substance. If it melts quickly it must be simple covalent.
- Add it to a circuit. Does the bulb light up as a solid? If so it is metallic (or graphite – you can check if it’s shiny in that case!)
- If it doesn’t conduct, dissolve and try again. If, when dissolved/melted, it does now conduct, it must be an ionic substance.
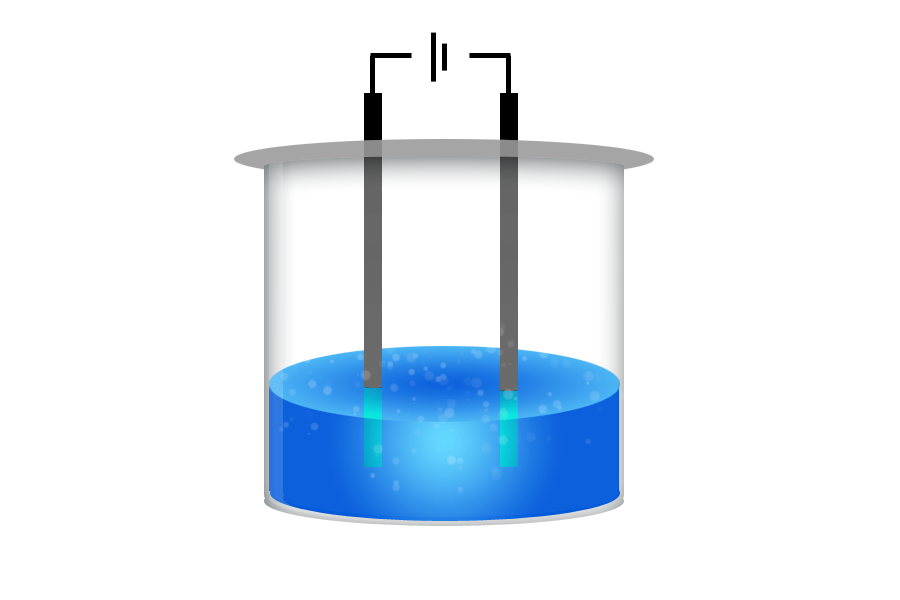
CH77: Explain the properties of the four types of bonding
Ionic
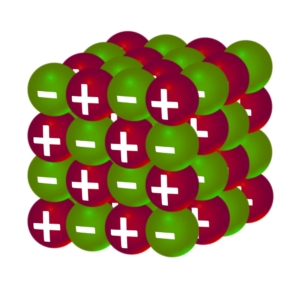
Property 1:
High Melting Point
Explanation:
Lots of energy is needed to break the strong electrostatic attraction between the cations and anions.
Property 2:
Only conduct electricity when molten
Explanation:
When solid, the ions are not free to move. When liquid/molten the ions are free to move.
Simple Molecular
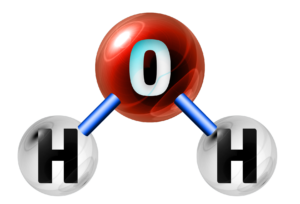
Property 1:
Low Melting Point
Explanation:
Not much energy to break the weak intermolecular forces.
Property 2:
Don’t conduct electricity
Explanation:
No spare/delocalised electrons to pass on a charge.
Giant Covalent
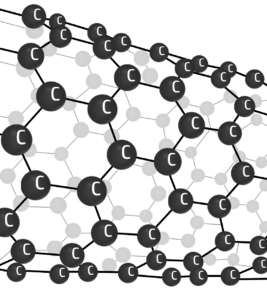
Property 1:
High Melting Point
Explanation:
Lots of energy is needed to break the strong covalent bonds.
Property 2:
Don’t conduct electricity (with exceptions)
Explanation:
No spare/delocalised electrons can flow to pass on a charge.
Exceptions: nanotubes/graphite/graphene: Delocalised electrons can flow and pass on a charge.
Metallic

Property 1:
High Melting Point
Explanation:
Lots of energy is needed to break the strong electrostatic attraction between the cations and delocalised electrons.
Property 2:
Do Conduct Electricity
Explanation:
Delocalised electrons can flow/move and pass on a charge.
