Ionic Bonding

CH48: Describe what an ionic bond is
All elements want a full outer shell. Metals do this by losing electrons and non-metals do this by gaining electrons.
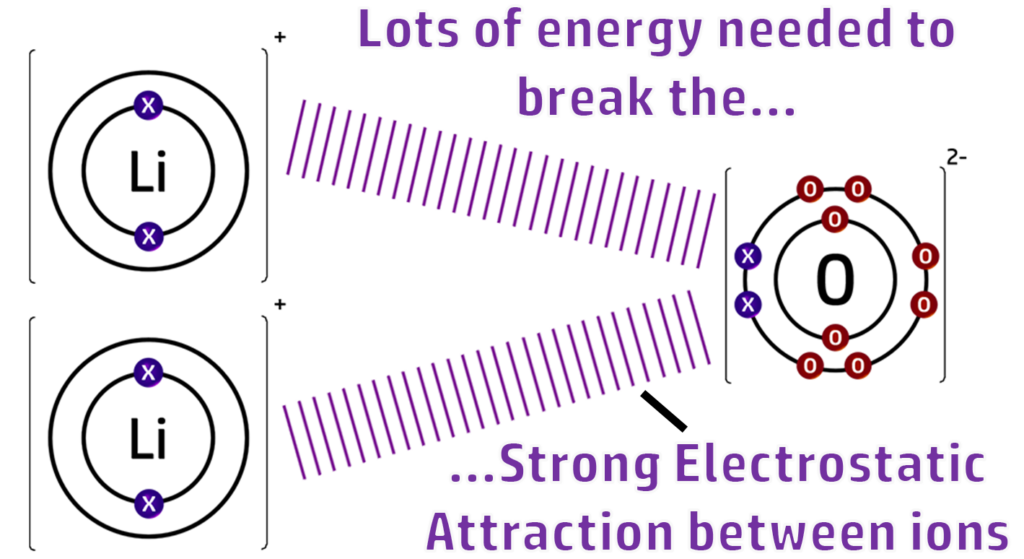
An ionic bond is the transfer of electrons from a metal to a non-metal. An ionic bond is the strong electrostatic attraction between the cation and anion

CH49: Describe what an ion is
An ion is any atom that has lost or gained electrons to become charged.
A metal will always lose electrons to become a positively charged cation (cations are paw-sitive!). A non-metal will always gain electrons to become an anion (ANegative ION)

CH50: Calculate the protons, neutrons and electrons for an ION
Calculating the number of protons and neutrons for an ion is exactly the same as for a normal atom.
For an ion, you must work out how many electrons an atom needs to gain/lose to get a full outer shell and take that away/add that to the number of electrons the atom would have.
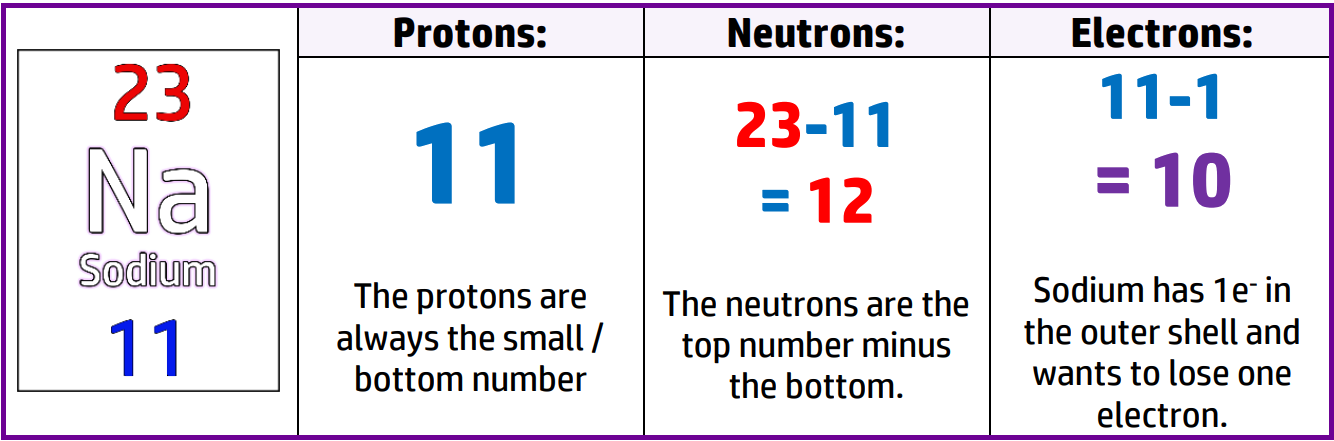
Clue: It will always be the same as the closest noble gas.
CH51: Describe the formation of ions in groups 1, 2, 6 and 7
Metals want to lose electrons to get a full outer shell. They always form positive cations.
Non-metals want to gain electrons to get a full outer shell. They always form negative anions.
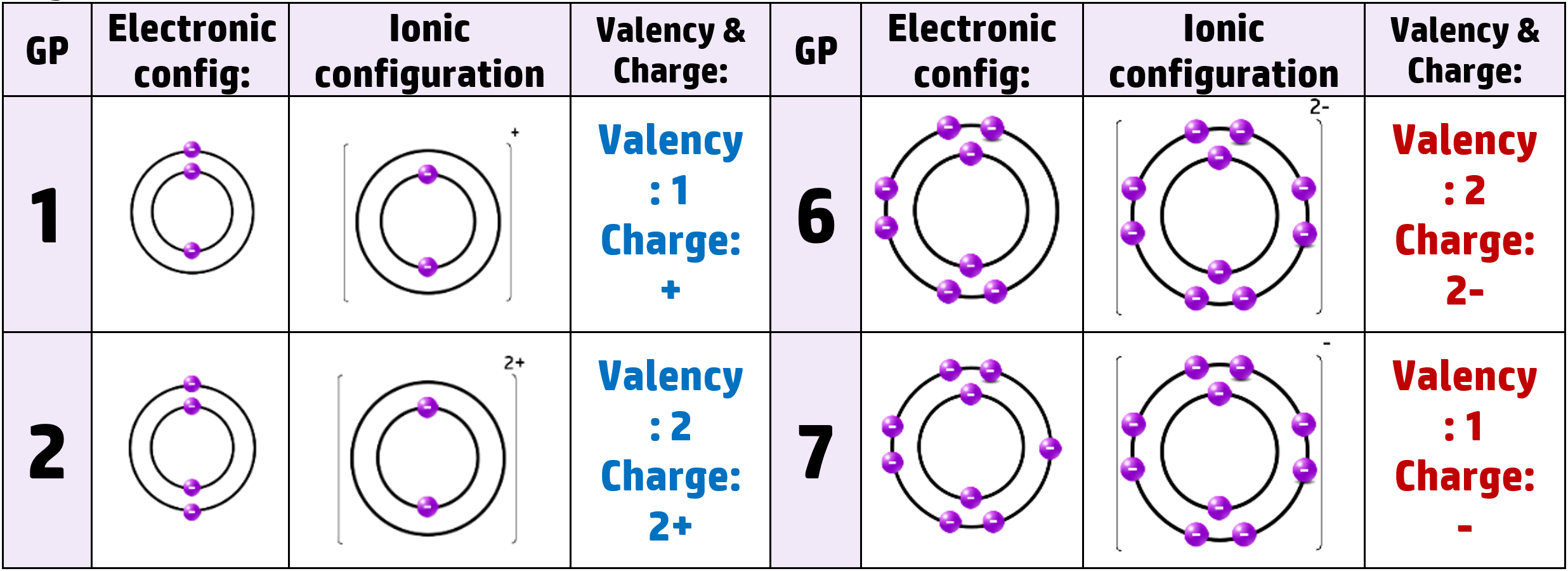
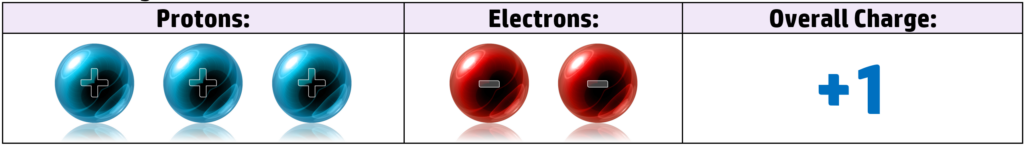
Lithium, for example, has 3 protons and 3 electrons. It has the electronic configuration of 2.1 – meaning it has 1 electron in the outer shell. When it loses that electron to get a full outer shell, it now has 3 positive protons and 2 negative electrons, giving it an overall charge of +1
Similarly, oxygen (in group 6) gains 2 negative electrons and becomes an O2- ion.
CH52: Use dot and cross diagrams to draw ionic bonding
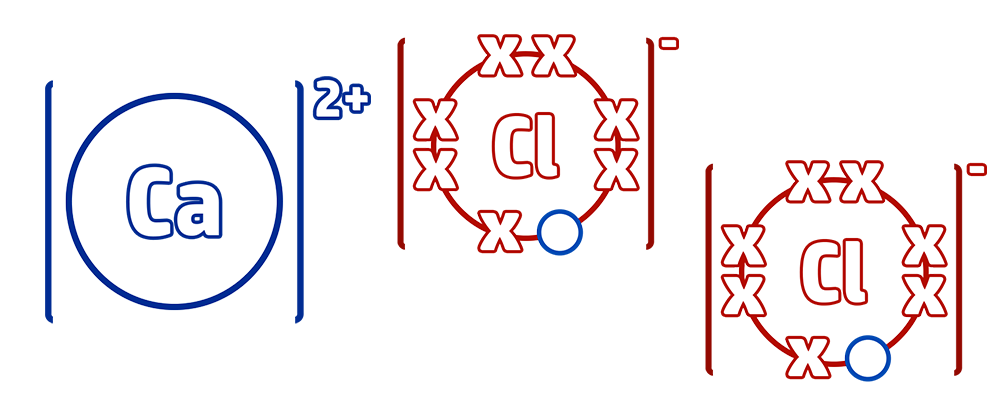
Step 1: When drawing ionic bonding start by drawing out the outer shell – which is the group the element is in. Calcium is in group 2 and chlorine is in group 7.

Step 2: Move as many electrons as possible from the metal to the non-metal by drawing arrows. Make sure that you don’t go below 0 for your metal, or above 8 for your non-metal.

Step 3: Continue this until both ions have a full outer shell. In this case, calcium still had 1 electron that it needed to lose, but calcium had a full outer shell – therefore add another calcium and continue to transfer.

Step 4: Redraw your new ions with their full outer shells. Add a bracket around both and add the charge (CH51) Calcium chloride has the formula CaCl2
CH53: Describe the difference between '-ide' and '-ate'
If you have a compound that ends in ‘-ide’, it will be made of a metal and a non-metal ONLY.
If you have a compound that ends in ‘-ate’, it will be made up of a metal, a non-metal and OXYGEN.
For example:
- Sodium sulphide is only made up of sodium and sulfur and has the formula Na2S.
- Sodium sulfate is made up of sodium, sulfur and oxygen and has the formula Na2SO4.
CH54: Work out the formula for ionic compounds
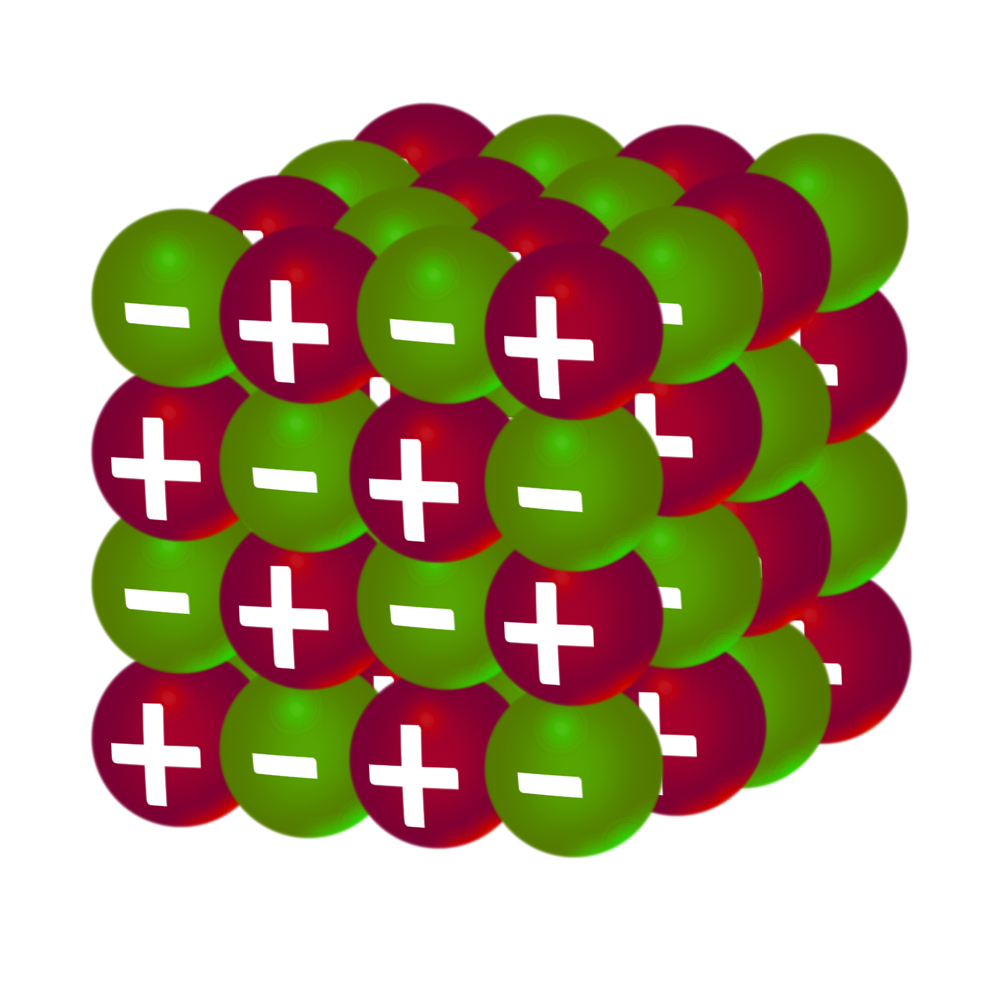
CH55: Describe what an ionic lattice is
A lattice is a regular arrangement of cations and anions held together by a strong electrostatic force of attraction.
CH57: Explain why ionic substances can only conduct when molten
Ionic Compounds cannot conduct when SOLID:
- When solid, there is a strong force of attraction between the ions.
- The ions are not free to move and cannot carry a charge.
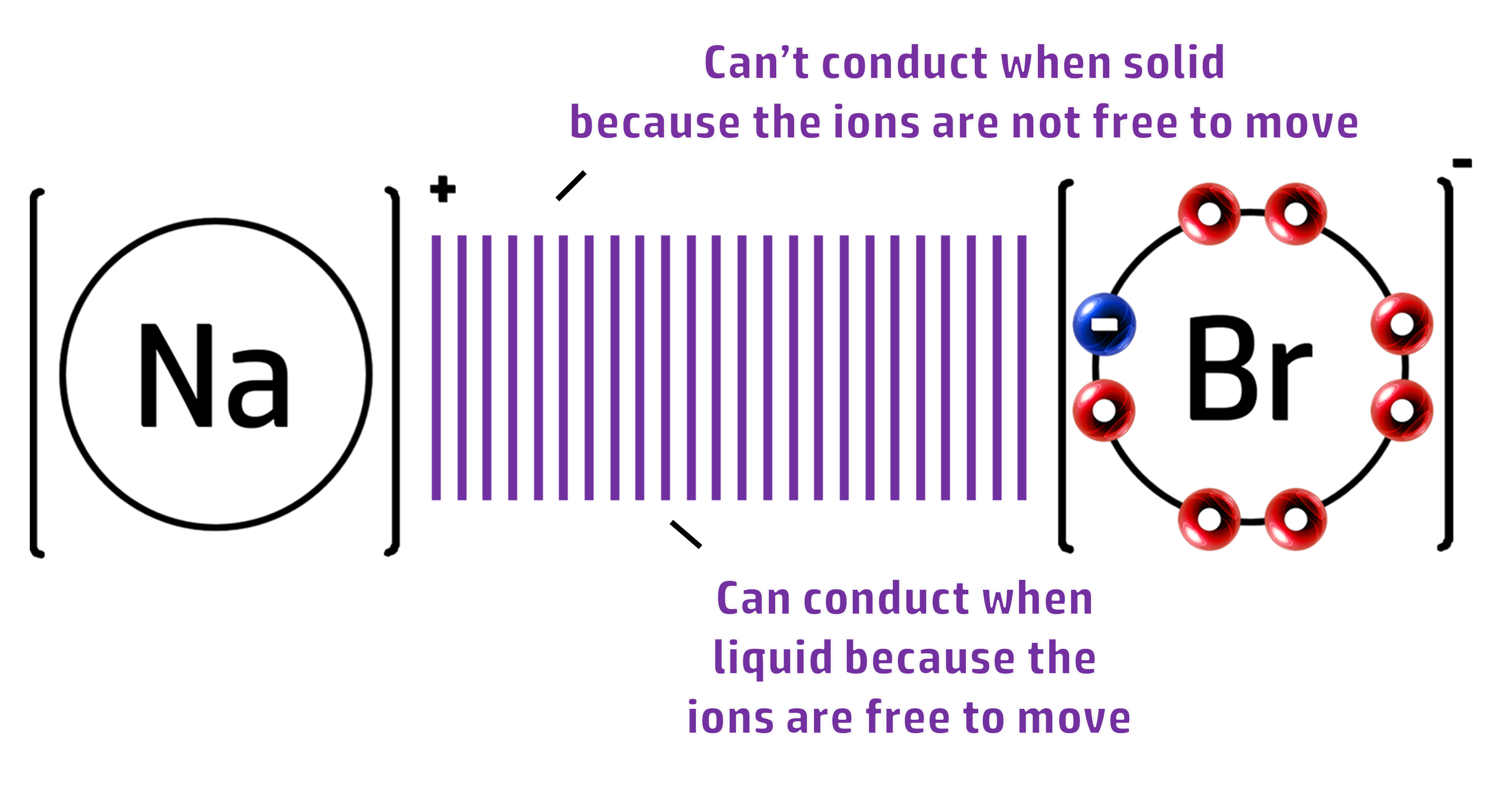
Ionic Compounds can conduct when LIQUID/MOLTEN/AQUEOUS:
- When molten (melted) or dissolved, the ions are free to move.
- The ions can now move and conduct electricity.