CHEMICAL CHANGE PRACTICALS

A GCSE chemistry revision page showing core chemical change practicals, including making salts from insoluble or soluble reactants and investigating pH changes with practical steps, key observations, and variables for experiments.
CH125: Describe how to make a salt from an insoluble reactant
If you want to make a salt from an insoluble reactant you can use filtration.
When you add enough of the insoluble reactant (e.g. copper oxide), the solution will become neutral.
If you add more copper oxide to the solution, the pH will not change because the copper oxide will not dissolve or react. Therefore, you can add in excess copper oxide to make sure the solution is neutral, then filter it to leave you with your neutral salt. See CH126 for more detail.
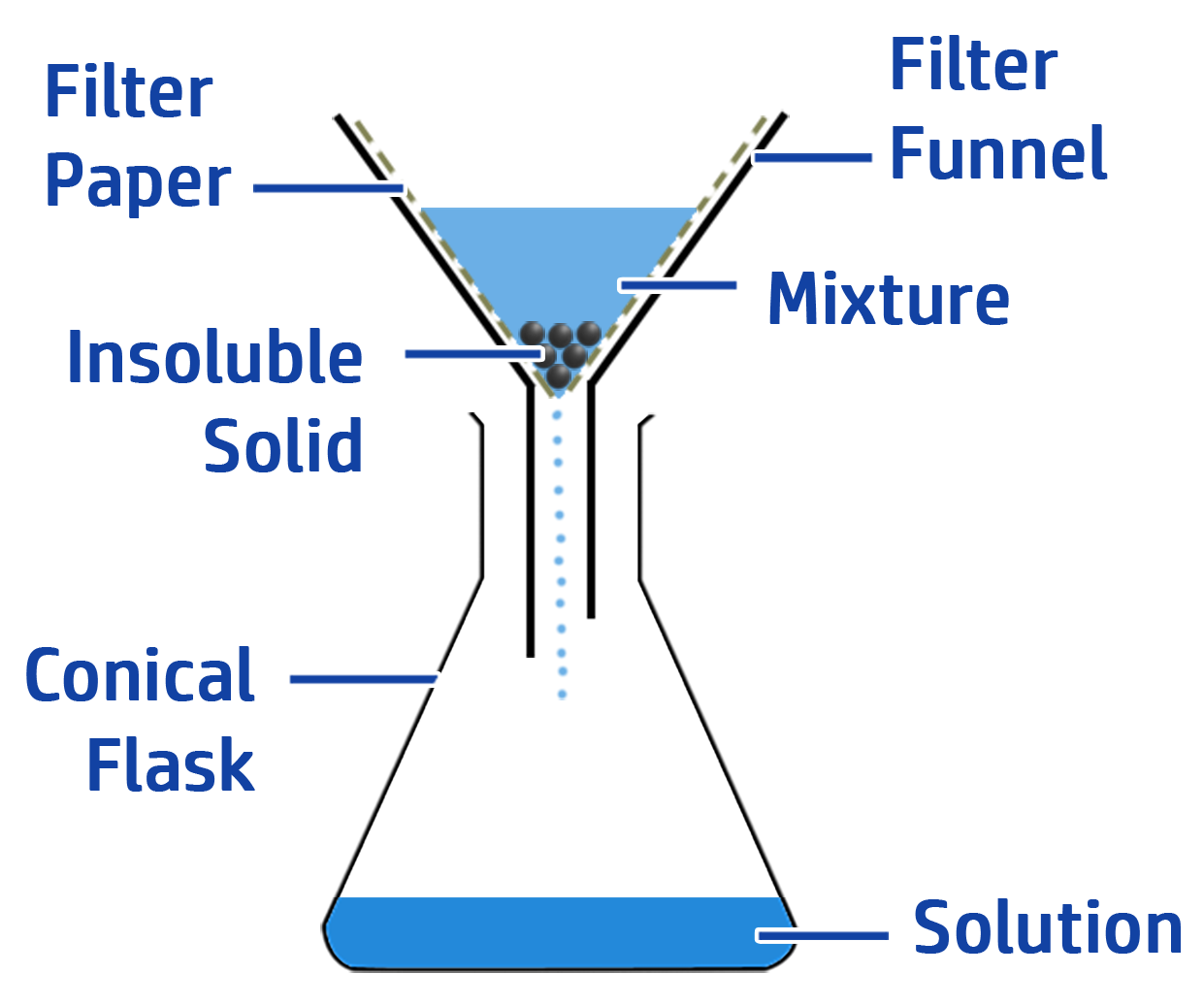
CH126: Core Practical: Preparing Copper Sulfate Crystals

Copper Oxide + Sulfuric Acid → Copper Sulfate + Water
Example: How can you produce soluble copper sulphate from sulphuric acid and insoluble copper oxide?
Key Steps: Mix → Neutral → Filter → Heat → Cool→ Dry
Phase One: Making the Copper Sulfate
- Mix the copper oxide with warm sulfuric acid (heating the acid speeds up the reaction).
- Continue until neutral. You can check this with pH paper.
- Filter the solution using a filter funnel and filter paper. This removes the excess copper oxide.

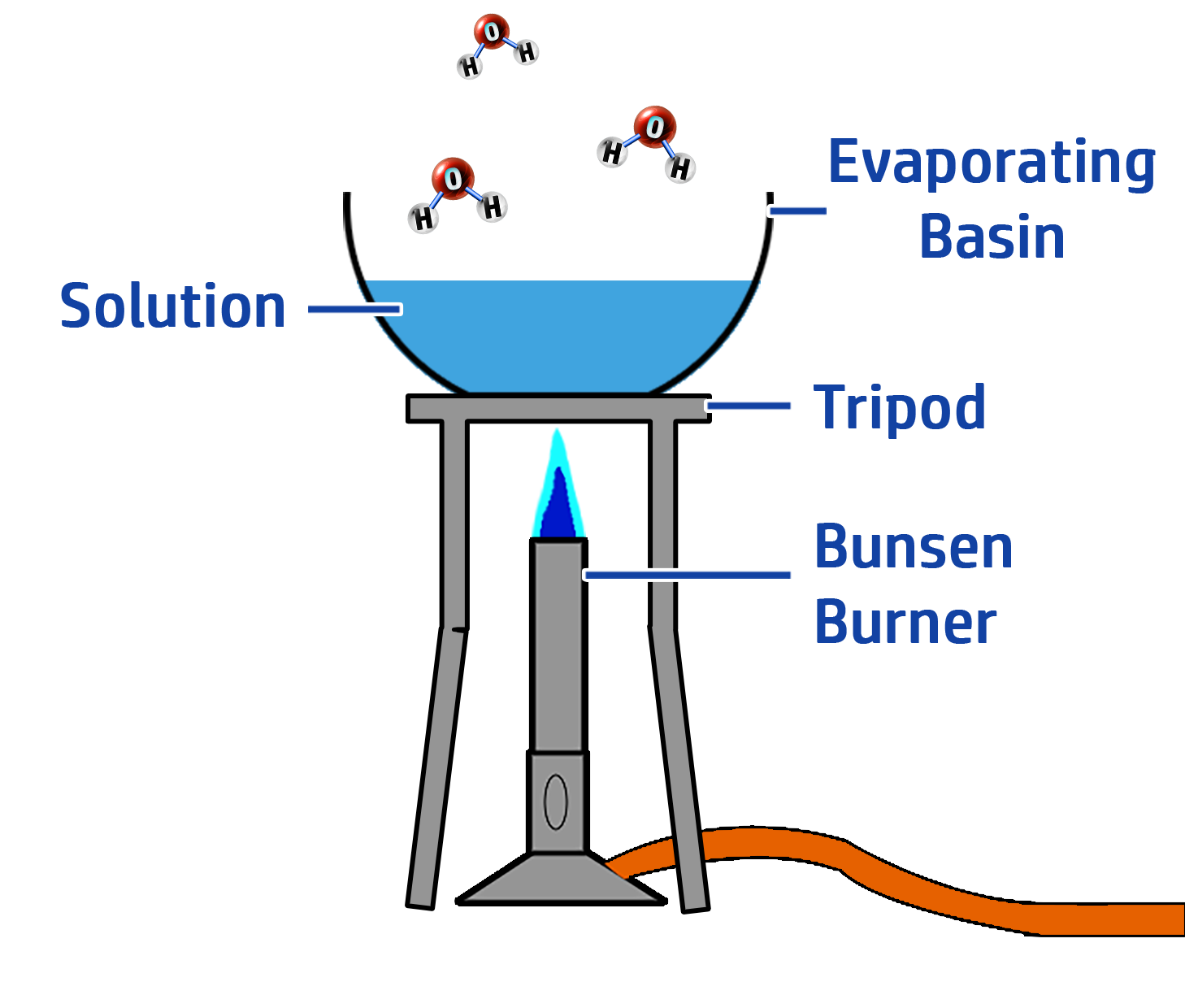
Phase Two: Preparing the Crystals
- Heat the solution and evaporate half of the water.
- Leave the solution to cool so that the rest of the water evaporates and crystals form.
- Dry the crystals between pieces of filter paper.

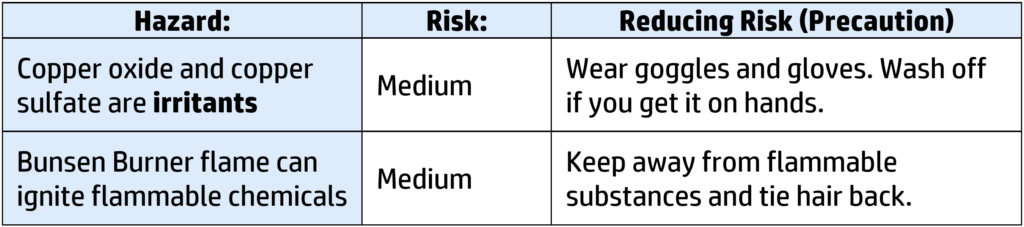
CH127: Core Practical: Investigating pH
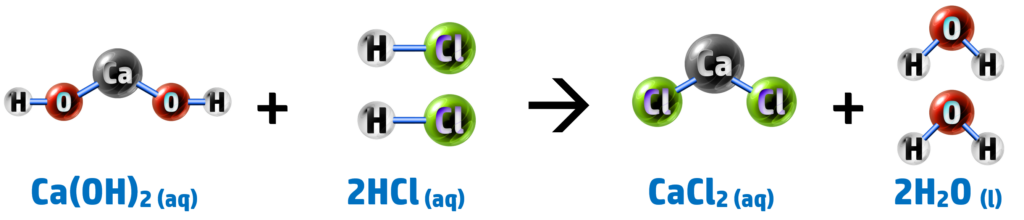
Calcium Hydroxide + Hydrochloric Acid → Calcium Chloride + Water
This is one of the big practicals that you need to know for the exam.
You could be asked how to carry it out; to analyse the results; to explain the results or to evaluate the risks. To investigate the pH of a substance, you will need to be able to do the following:
Key Steps: pH → 0.3g → Mix → pH → Neutral
Method
- Measure the pH of 50cm3 of hydrochloric acid using a pH probe (or pH paper).
- Measure out 0.3g of calcium hydroxide and add it to the beaker.
- Stir the beaker to mix the chemicals and make sure they are fully reacted.
- Check the pH of the substance again and record it in a table.
- Repeat until the pH of the solution is above neutral.

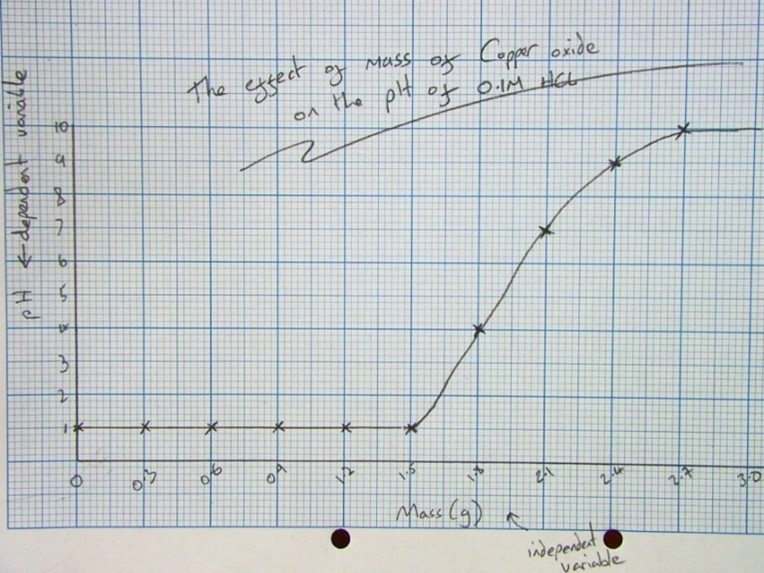
Analysing Results
- Once you have a graph of results drawn, you can draw a line across and then down from pH 7.
- From this graph, it took 2.1g of Calcium Hydroxide to completely neutralise the hydrochloric acid.
Explaining the results:
- The pH starts low as there are lots of H+ ions (it is acidic).
- It starts to rise as the hydroxide (an alkali) neutralises the acid.
- The pH continues to rise above 7 as calcium hydroxide is soluble, so the solution contains OH- ions making it alkaline.
Variables:
- Independent variable (the thing you change!): The mass of calcium carbonate, Ca(OH)2.
- Dependent variable (the thing you measure!): The pH of substance.
- Control variables (the things you keep the same!): The volume of acid, the concentration of acid, the type of tablet, the surface area of the tablet.

CH128: Describe how to make a salt from a soluble reactant
If you want to make a salt from a soluble reactant you cannot use filtration.
As you add the acid to your soluble alkali, it will reach pH 7 (neutral), but the pH will continue to rise because the alkali will start being added to your neutral solution.
To get round this, you need to use a titration (see CH129). You need to add the acid to the soluble alkali until it is exactly neutral (you can do this using an indicator) – at which point you can evaporate off the water, leaving your crystals behind.
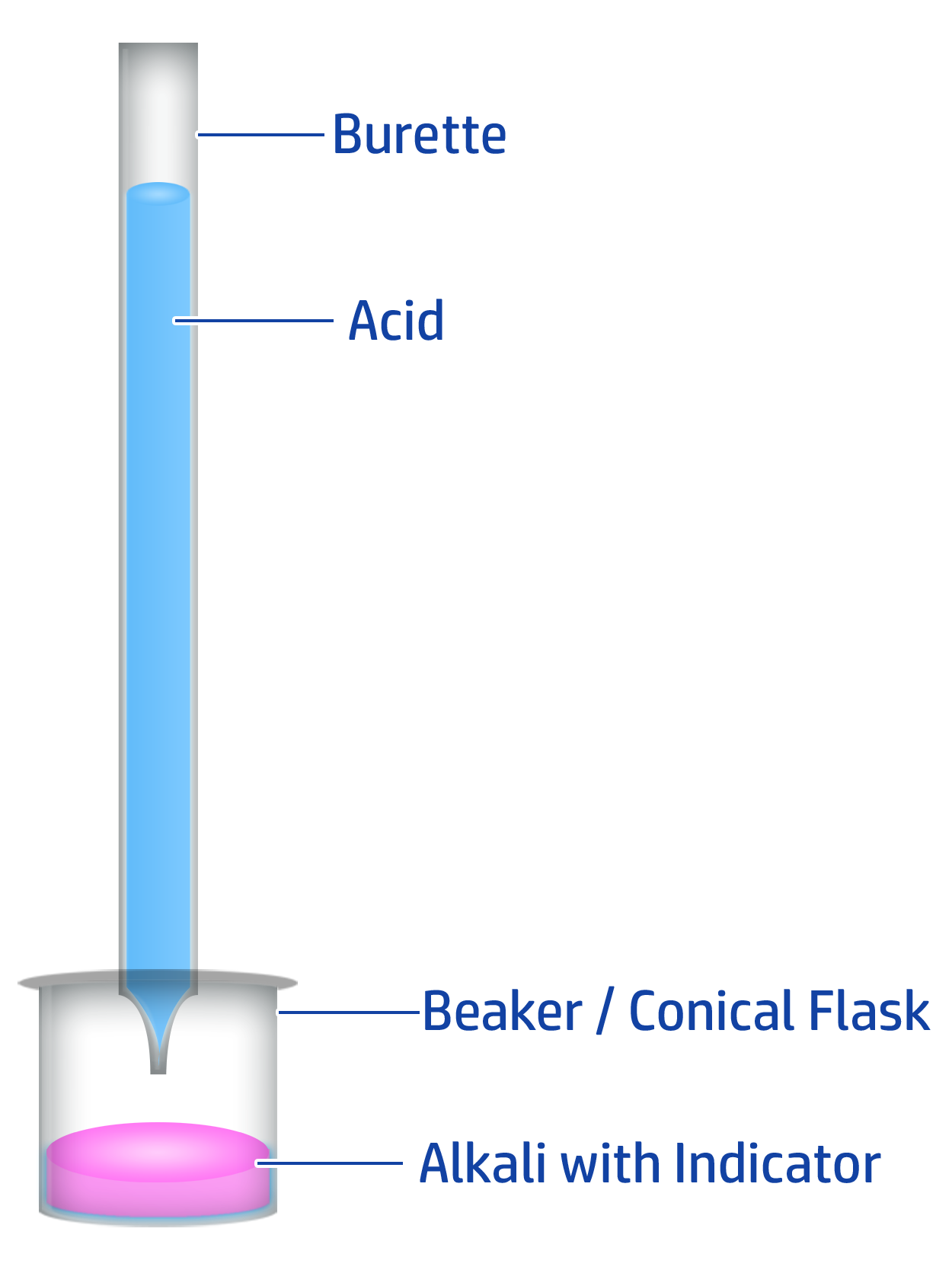
CH129: How to carry out a titration to produce a pure, dry salt
Key Steps: Burette → Pipette → Indicator → End Point
- Fill a burette to the 0.0cm3 line with hydrochloric acid.
- Add 20cm3 of your alkali (sodium hydroxide) into a conical flask using a pipette.
- Add a few drops of phenolphthalein indicator - the alkali will turn pink.
- When close to the end point, add the acid drop by drop until the indicator changes from pink to colourless.
If you are asked to produce a pure dry salt from this titration, do the following extra steps:
- Repeat the experiment without the indicator, this gives you just sodium chloride and water (pure).
- Heat the solution gently to evaporate half of the water.
- Leave the solution to cool forming crystals of sodium chloride
- Leave on a windowsill to dry.
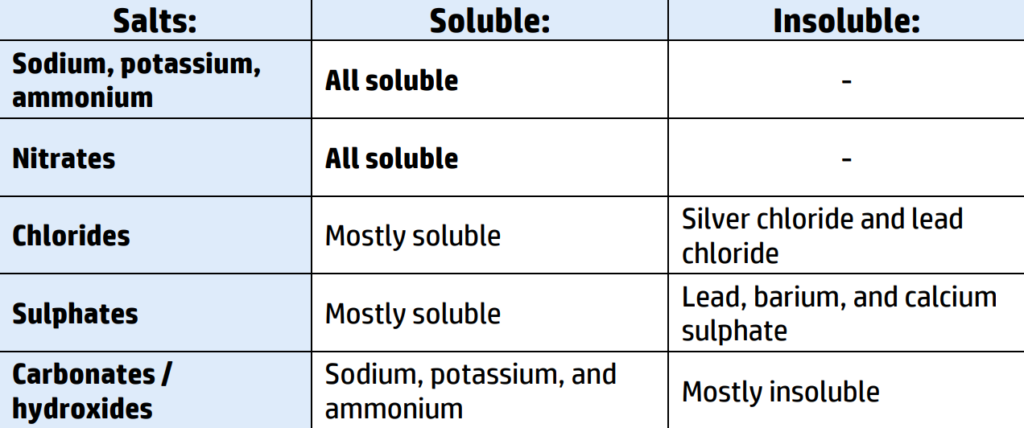
CH130: Recall the solubility rules
CH131: Predicting and naming precipitates
If you are given a word equation and asked to identify the name of the precipitate (insoluble solid) you need to look at the solubility rules (CH130)
Example: State the name of the precipitate formed in the following reaction:
Silver nitrate + copper chloride → silver chloride + copper nitrate
Start by looking at the products.
You can see from the solubility table in CH130 that most chlorides are soluble except for silver chloride and lead chloride.
Therefore, silver chloride is insoluble = your precipitate!
You then check the other product – copper nitrate - you can see that all nitrates are soluble, so copper nitrate will remain a liquid.
CH132: Describe how to produce a pure, dry precipitate
Key Steps: Dissolve → Mix → Filter → Wash → Dry
If you wanted to prepare a pure dry precipitate of lead chloride from the above reaction, there are 4 steps you need to follow:
- Dissolve the solid copper chloride and lead nitrate.
- Mix them together to produce your copper nitrate and lead chloride.
- Filter the solution to give you lead chloride in the filter paper.
- Wash the filter paper to remove the soluble copper nitrate.
- Dry the filter paper in a desiccator / oven to leave you with your pure, dry precipitate.

