ACIDS, BASES AND NEUTRALISATION
CH109: Identify the ions that make something acidic

Acids always contain H+ ions. Examples include hydrochloric acid, HCl, nitric acid, HNO3, and sulfuric acid, H2SO4.
Alkalis usually contain OH- ions. Examples include sodium hydroxide, NaOH, magnesium oxide, Mg(OH)2 and aluminium hydroxide, Al(OH)3
CH110: Identify the pH of acids and alkalis
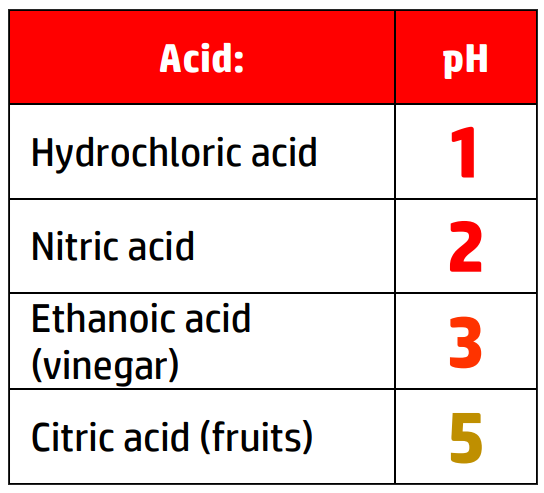
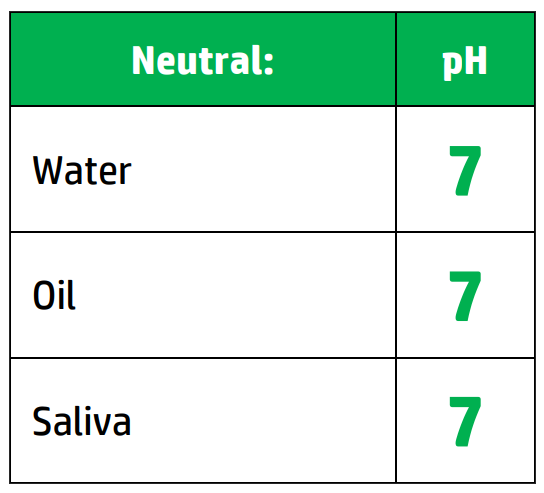
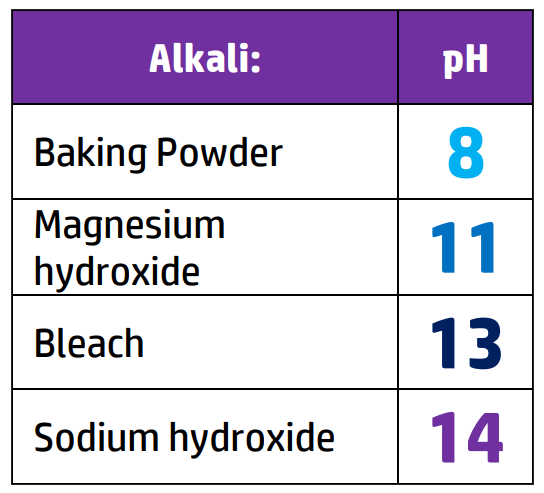
CH111: Identify the colour changes in different indicators
| Indicator | Colour in acid | Colour when neutral | Colour in alkali |
|---|---|---|---|
| Phenolphthalein | Colourless | Colourless | Pink |
| Methyl Orange | Red | Orange | Yellow |
| Litmus Solution | Red | Purple | Blue |
CH112: Describe the terms 'dilute' and 'concentrated'
Dilute
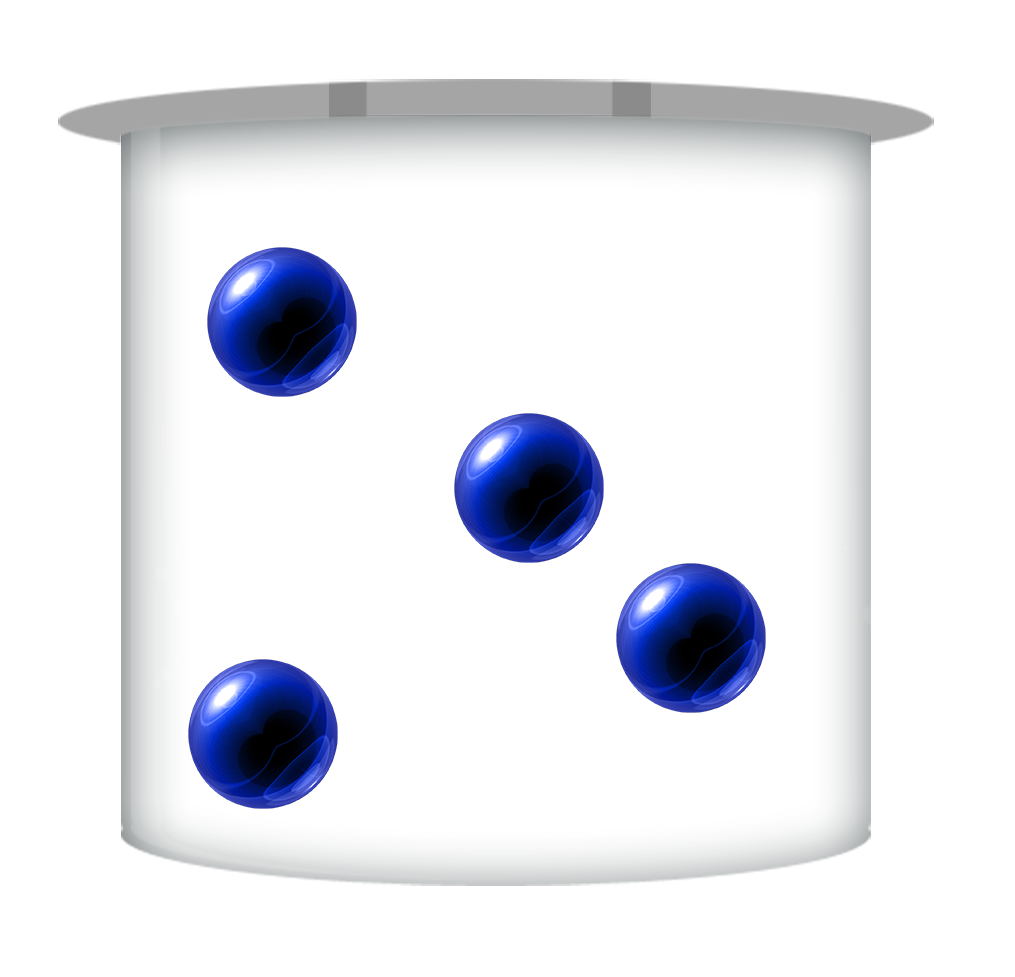
The more dilute an acid is, the less H+ ions there are per litre (dm3)
Concentrated
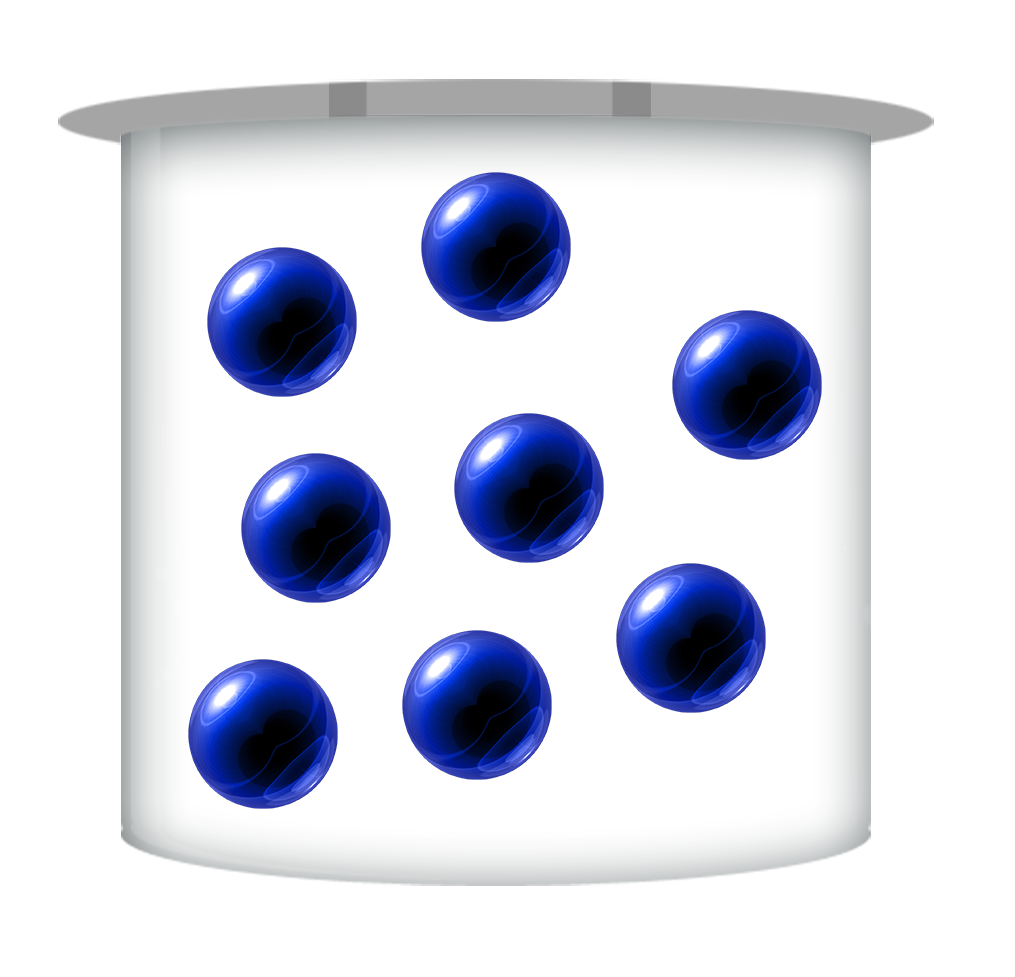
The more concentrated an acid is, the more H+ ions there are per litre (dm3)
CH113: Explain the difference between a weak and a strong acid
Strong Acids
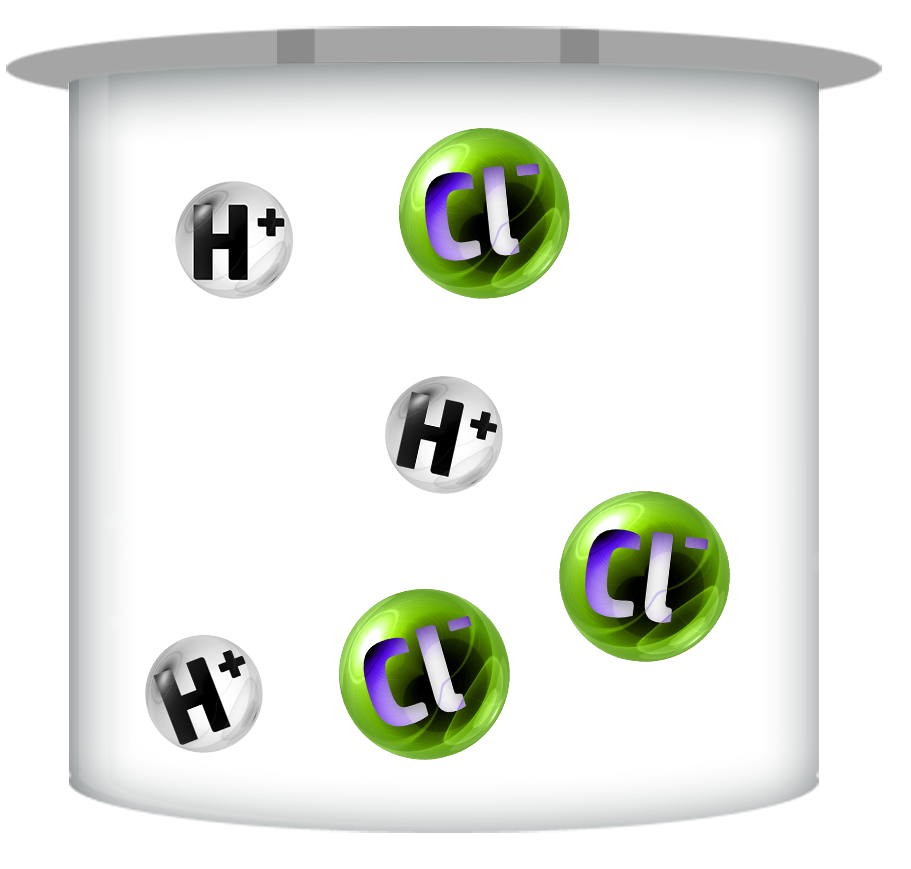
Strong acids dissociate / ionise (split up) completely – producing more H+ ions.
They have lower pH’s (0-2)
Weak Acids
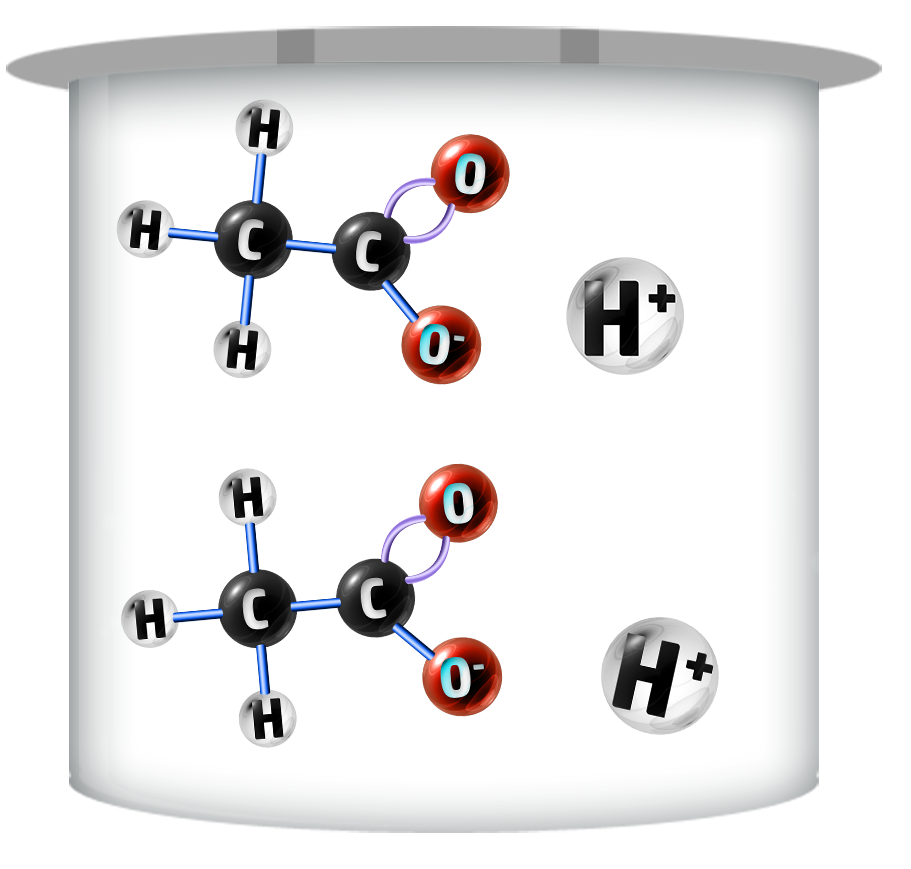
Weak acids do not fully ionise – producing fewer H+ ions.
Their pH ranges are higher (3-6)
CH114: Describe what a base is
A base is a chemical compound that can react with an acid and neutralise it.
There are two types of bases:
- Oxides/Hydroxides which produce a salt and water.
- Carbonates which produce a salt, carbon dioxide and water.
CH115: Explain the difference between an alkali and a base
Similarity: Both bases and alkalis neutralise acids.
Difference: Alkalis are soluble. Bases can be both soluble or insoluble.
Example:
- Sodium hydroxide and copper oxide both neutralise acids, so are both bases.
- Sodium hydroxide is soluble, so it is a base and an alkali.
- Copper oxide is insoluble, so it is a base, but not an alkali.
CH116: Write word equations for reactions between metals and acids
All acids react with metals to produce a salt and hydrogen:

Step 1: To name the salt, take the name of the metal (sodium) and add the salt ending, which you get from the acid:
- Hydrochloric acid produces a chloride salt.
- Nitric acid produces a nitrate salt.
- Sulfuric acid produces a sulfate salt.
Here we have nitric acid, so our salt becomes sodium nitrate.
Step 2: If you only have a metal (i.e. no hydroxide or carbonate), simply add hydrogen to the end of the word equation.
If you have a hydroxide, see CH117 and if you have a carbonate look at CH118.
CH117: Write word equations for reactions between metal hydroxides and acids
All acids react with metal hydroxides to produce a salt and water:
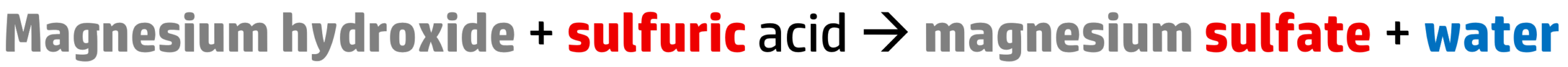
Step 1: To name the salt, take the name of the metal (magnesium) and add the salt ending, which you get from the acid:
- Hydrochloric acid produces a chloride salt.
- Nitric acid produces a nitrate salt.
- Sulfuric acid produces a sulfate salt.
Here we have sulfuric acid, so our salt becomes magnesium sulfate.
Step 2: If you have a metal hydroxide (i.e. magnesium hydroxide), simply add water to the end of the word equation.
If you just have a metal (no hydroxide or carbonate), see CH116 and if you have a carbonate look at CH118.
CH118: Write word equations for reactions between metal carbonates and acids
All acids react with metal carbonates to produce a salt, water AND carbon dioxide:

Step 1: To name the salt, take the name of the metal (calcium) and add the salt ending, which you get from the acid:
- Hydrochloric acid produces a chloride salt.
- Nitric acid produces a nitrate salt.
- Sulfuric acid produces a sulfate salt.
Here we have hydrochloric acid, so our salt becomes calcium chloride.
Step 2: If you have a metal carbonate (i.e. calcium carbonate), simply add water + carbon dioxide to the end of the word equation.
If you just have a metal (no hydroxide or carbonate), see CH116 and if you have a hydroxide look at CH117.
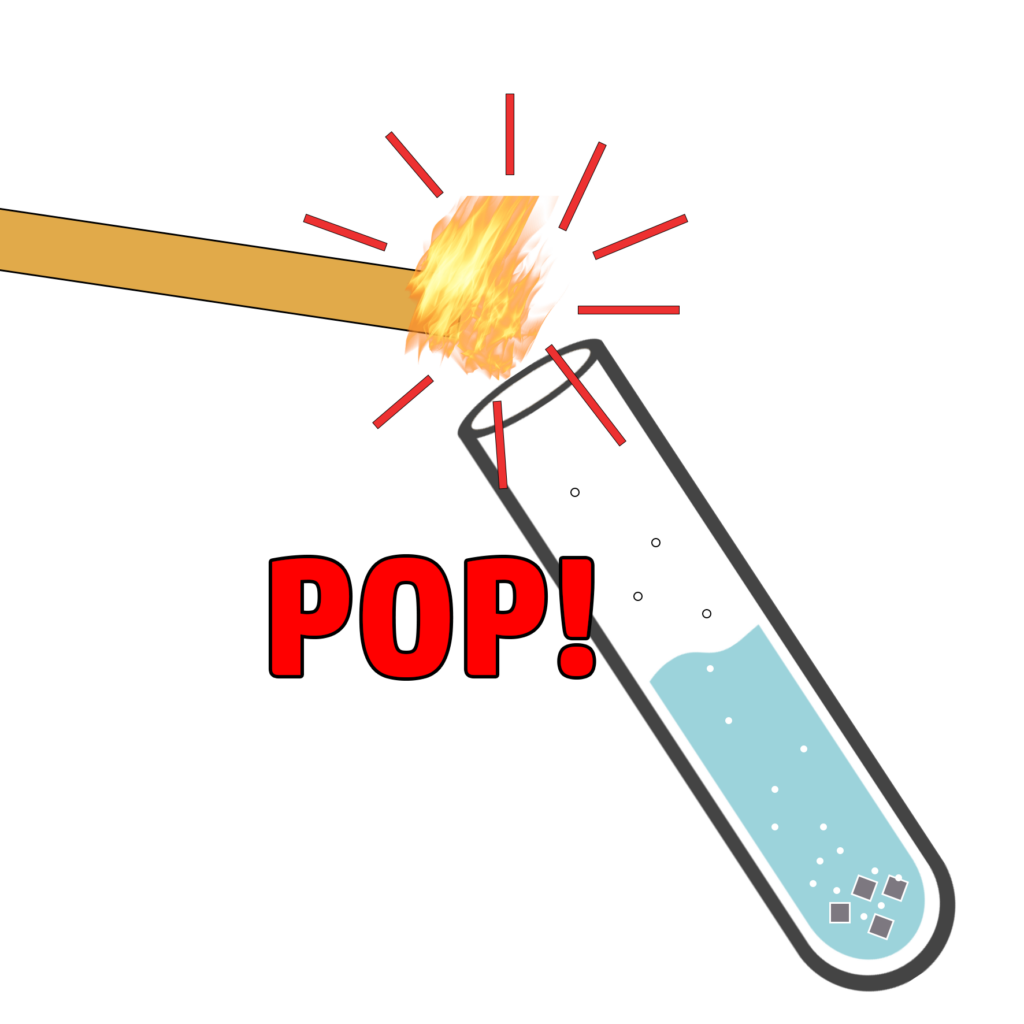
CH119: Describe the test for hydrogen
Hydrogen is a flammable gas.
If you take a lit splint and add it to a test tube containing hydrogen, you will hear a squeaky pop.
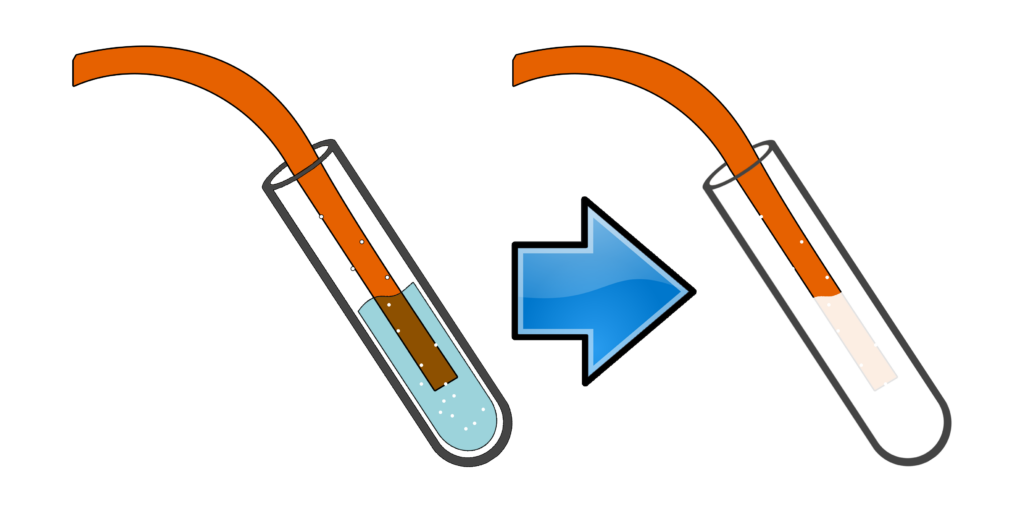
CH120: Describe the test for carbon dioxide
Carbon dioxide turns limewater cloudy.
Bubble the gas through limewater and if it goes cloudy/milky, carbon dioxide is present.
The carbon dioxide reacts with the limewater (calcium hydroxide) to form calcium carbonate - a white solid.
CH121: Describe what is seen during neutralisation reactions
The clue is in the state symbols. Look at the chemical equation below. What would you be able to see during this reaction?

From this reaction, you would be able to see two things:

You have a solid (s) on the left…but not on the right. You would see it disappear / dissolve
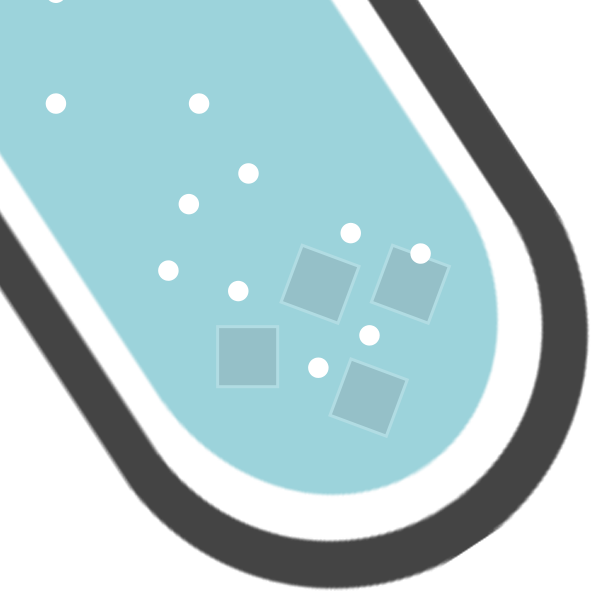

You have a gas (g) on the right…you can’t say you would see a gas! You would see bubbles/fizzing!
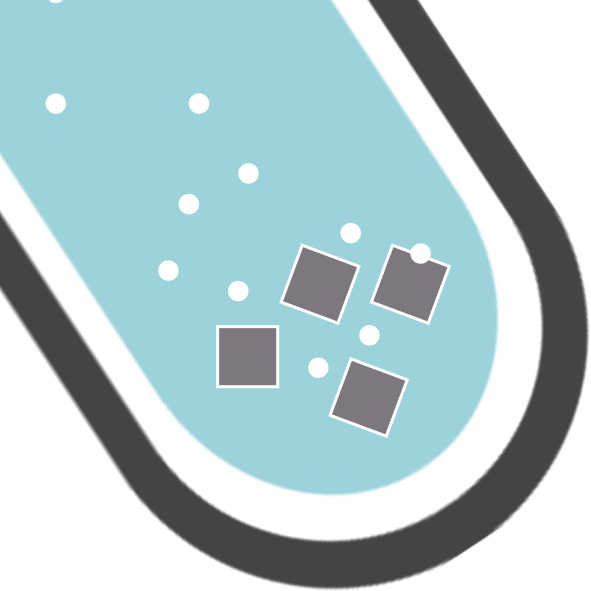
CH122: Explain, in terms of ions, what is happening during neutralisation
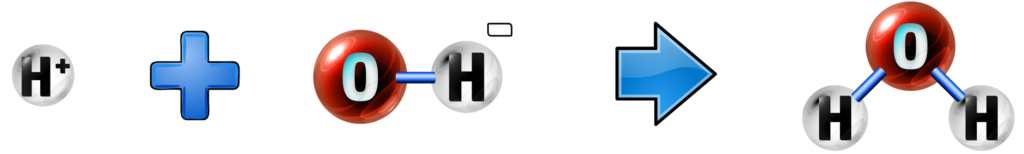
Every time a hydroxide (an alkali containing OH- ions) reacts with an acid (which contains H+) ions, the H+ and OH- ions react together to form water.
This happens in every acid-hydroxide reaction, giving the ionic equation of:
H+ + OH- → H2O
Every time a carbonate (a base containing CO32- ions) reacts with an acid (which contains H+) ions, the H+ and CO32- ions react together to form carbon dioxide and water.
This happens in every acid-carbonate reaction, giving the ionic equation of:
H+ + CO32- → CO2 + H2O

CH123: Link the number of H+ ions to the concentration of an acid
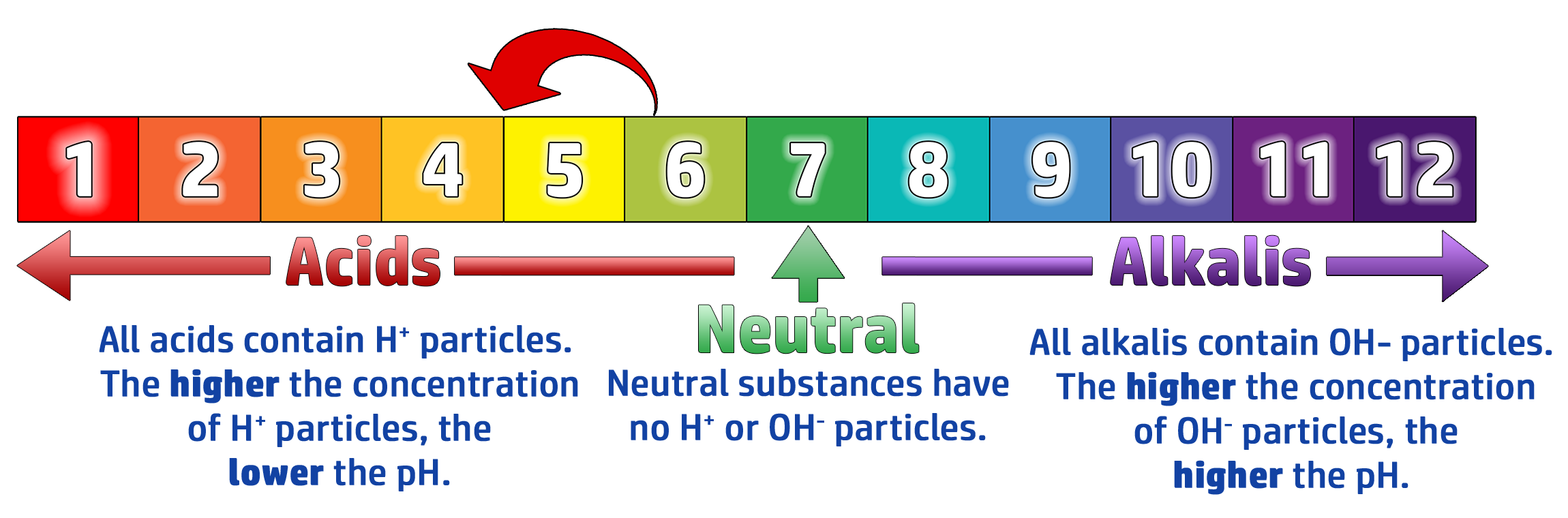
If you increase the number of H+ ions, the pH will go down towards 1.
If you increase the number of OH- ions, the pH will go up towards 14.
CH124: Calculate the change in pH and concentration
Every time the pH decreases by one, the concentration of H+ ions increases by 10 times.
Example: Calculate how much more concentrated hydrochloric acid is, with a pH of 0, than ethanoic acid, with a pH of 4.
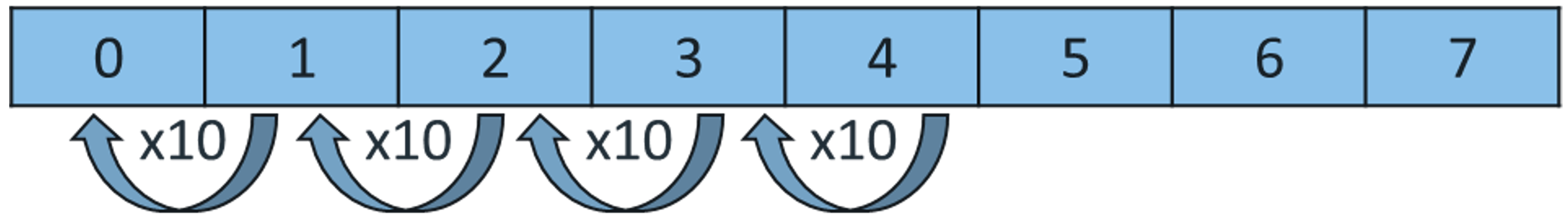
As you can see, the pH has gone down by 4, so it is 10 x 10 x 10 x 10 times more concentrated = 10,000 times more concentrated.
