RATES AND ENERGY CHANGES

A GCSE revision page on rates of reaction and energy changes including practical methods for measuring rate, collision theory, factors affecting rate, interpreting rate graphs, catalysts, activation energy, reaction profiles, and calculations of rate and energy change.
CH197: Core Practical: Investigating Rates of Reaction
Part 1: Measuring the production of a gas (in the reaction between hydrochloric acid and marble chips)
Hydrochloric acid + calcium carbonate → calcium chloride + carbon dioxide + water
2HCl (aq) + CaCO3 (s)→ CaCl2 (aq) + CO2 (g) + H2O (l)
Key Steps: Mix → Bung → Timer → Gas → Repeat
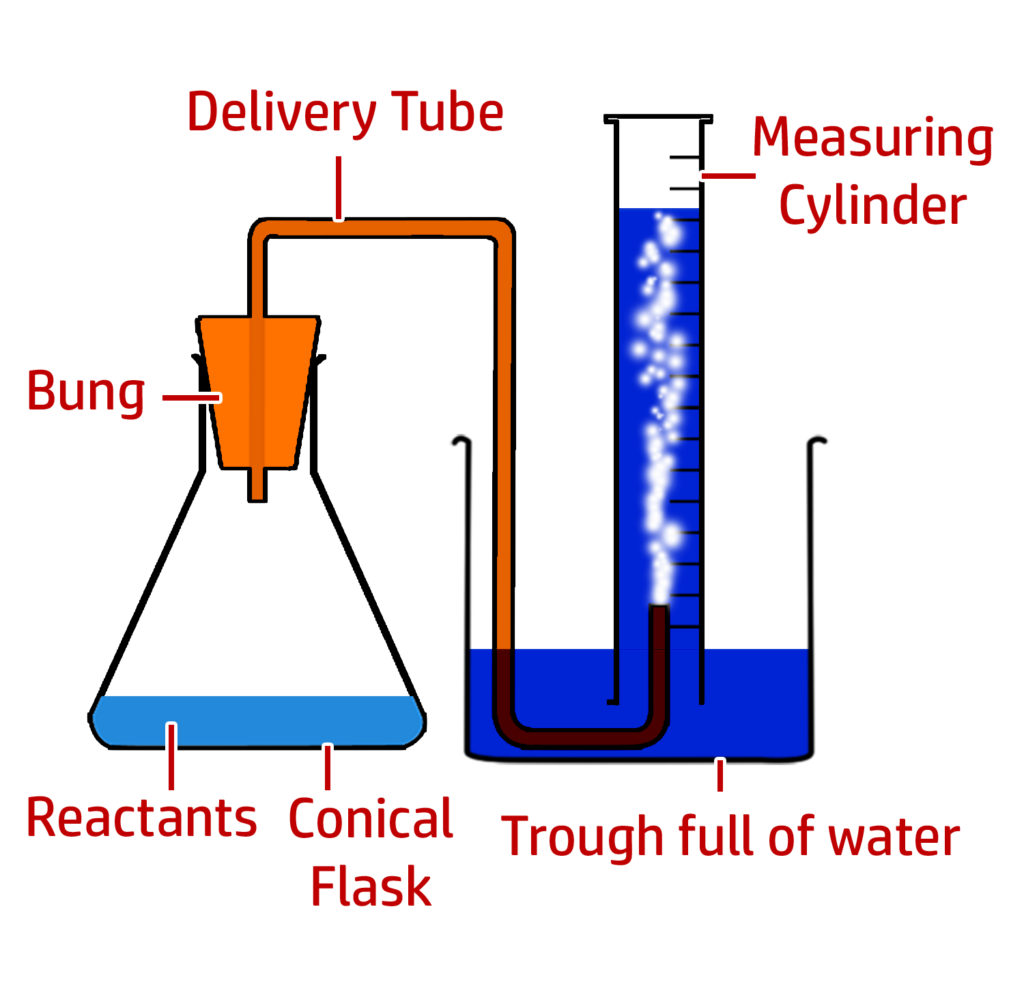
Method:
- Mix the hydrochloric acid and marble chip.
- Add the bung to the conical flask and start the timer.
- Stop the timer when 50cm3 of carbon dioxide gas has been produced.
- Repeat for different {concentrations / temperatures / surface areas}
Variables:
- Independent Variable: Change the concentration, surface area OR temperature (the question will give you this info).
- Dependent Variable: Measure the time it takes to produce 50cm3 of gas.
- Control Variable: Keep the volume of acid and mass of marble chip the same (as well as the {concentration/surface area/temperature} that wasn’t changed earlier.
Results:
Increasing the {temperature / concentration / surface area} will mean a faster reaction. This will mean that carbon dioxide is produced quicker so the {water is displaced quicker / gas syringe fills up quicker}.
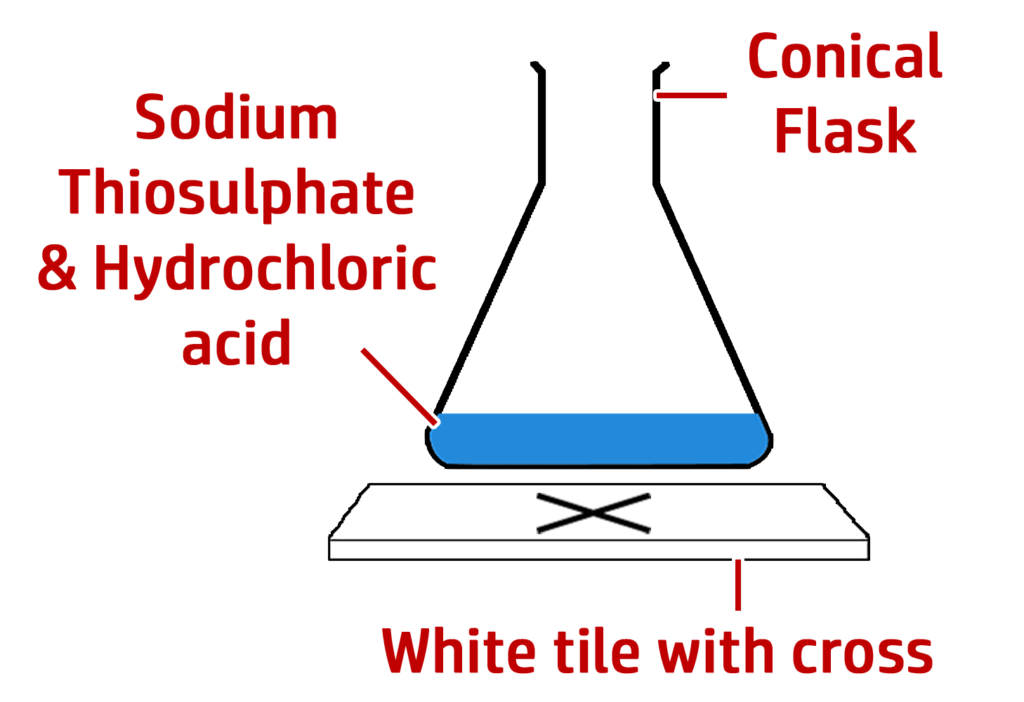
Part 2: Investigating the disappearing cross:
Hydrochloric acid + sodium thiosulfate → sodium chloride + sulfur dioxide + sulfur + water
2HCl (aq) + Na2S2O3 (aq)→ 2NaCl (aq) + SO2 (g) + S (s) + H2O (l)
Key Steps: Cross → Mix → Timer → Disappear→ Repeat
Method:
- Draw a cross on a white tile.
- Mix the acid and sodium thiosulfate and start the timer.
- Stop the timer when the cross disappears.
- Repeat for different {concentrations / temperatures}
Variables:
- Independent Variable: Change the concentration or the temperature.
- Dependent Variable: Measure the time it takes for the cross to disappear.
- Control Variable: Keep the volume of the acid and sodium thiosulfate the same, as well as whichever you didn’t change out of temperature/concentration.
Results:
Increasing the {temperature / concentration} will mean a faster reaction. This will mean that solid sulfur is produced quicker so the cross will disappear quicker.
CH198: Identify what is needed for a reaction to occur
For a chemical reaction to occur, two things need to happen:
- A collision between your reactant particles
- The collisions need to have enough energy to break bonds in the reactants. This is called the Activation Energy.
If a collision occurs between two particles without enough energy, there will be no reaction – therefore no products will be formed.
CH199: Collision Theory: Explain how to speed up a chemical reaction
Increasing Temperature:
If you increase the temperature, the particles have more kinetic energy.
There will be more successful collisions every second – meaning a faster rate of reaction.
Increasing Concentration:
If you increase the concentration, there are more particles in the same volume.
This means there are more frequent collisions – increasing the rate of reaction.
You can also increase the pressure, which means there particles will be closer together, meaning more frequent collisions.
Increasing Surface Area:
If you increase the surface area of a solid, you break it up into smaller pieces.
This means that there is a larger surface area-to-volume ratio – more solid is exposed to the acid.
This will mean that there are more frequent collisions and a faster rate of reaction.
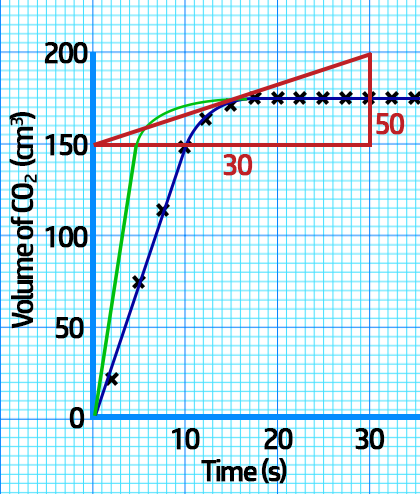
CH200: Interpreting Rate Graphs
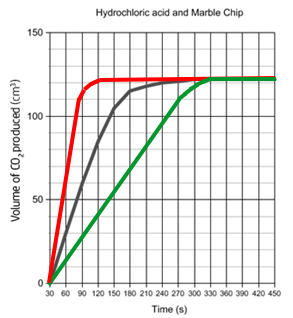
Sketching changing conditions:
If you increase the temperature / concentration / surface area, the reaction will be quicker, and the rate graph will be steeper as shown in red below.
If you decrease the temperature / concentration / surface area, the reaction will be slower, and the rate graph will be shallower as shown in green on the left.
Note that in both situations, the total volume at the end is always the same – the flat bit should always be at the same level.
The reason the rate of reaction decreases is because, as the reaction proceeds, there are less reactants, meaning less frequent collisions.
Rate calculations:
You can be asked two different ways of calculating the rate of reaction:
1. Calculating the mean / average rate of reaction: The mean rate of reaction is the average rate at a specific time. To calculate this, all you need is the time and the volume.
Example: In the first 50s of a reaction, 25cm3 of carbon dioxide was produced. Calculate the mean rate.
- Mean rate = Volume (cm3) ÷ Time (s)
- Mean rate = 25cm3 ÷ 50s = 0.5cm3/s.
2. Calculating the rate of reaction at a SPECIFIC time: To calculate this, you need to draw a tangent at the time given and then divide the change in volume (cm3) by the change in time (s).
Example: Calculate the actual rate of CO2 production at 15s.
From the tangent, you can see that at 15 seconds:
- The change in volume = 200cm3 - 150cm3 = 50cm3.
- The change in time = 30s - 0s = 30s
- Actual rate = Change in volume (cm3) ÷ Change in time (s)
- Actual rate = 50cm3 ÷ 30s = 1.7cm3/s.
CH201: Describe how to investigate temperature changes
Key Steps: Start → Mix → Final → Change
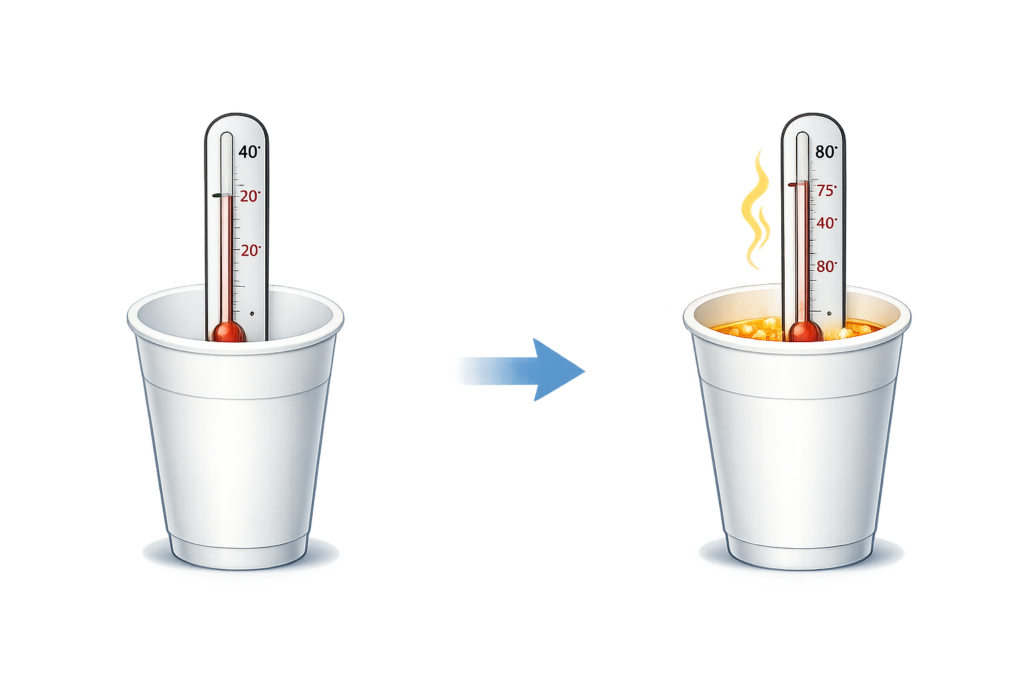
- Measure the starting temperature using a thermometer.
- Mix the chemicals and wait for the reaction to finish / the temperature to stop changing.
- Measure the final temperature and calculate the change in temperature.
- If the temperature increases, it is an exothermic reaction (e.g. neutralisation and displacement).
- If the temperature decreases (e.g. dissolving salts and photosynthesis), it is an endothermic reaction (See CH202).
CH202: Describe exothermic and endothermic reactions in terms of heat and temperature

Exothermic:
- In an exothermic reaction, heat is given out to the surroundings.
- This means that:
- The temperature increases.
- Heat energy is given out to the surroundings (so the surroundings get hotter).
- The products have less energy than the reactants. (See CH203)

Endothermic:
- In an endothermic reaction, heat is taken in from the surroundings.
- This means that:
- The temperature decreases.
- Heat energy is taken in from the surroundings (so the surroundings get colder).
- The products have more energy than the reactants. (See CH203)
CH203: Draw reaction profiles for exothermic and endothermic reactions
EXOTHERMIC
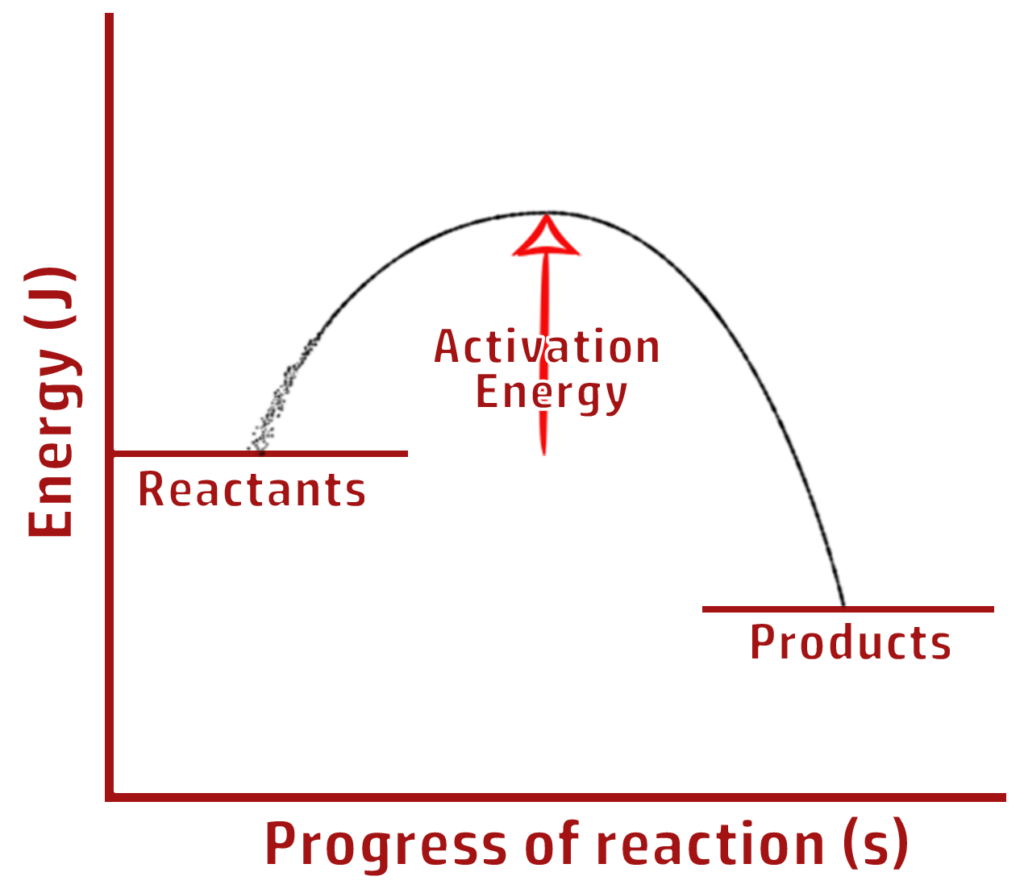
Clue: More people have exited the shop (products have less energy) so it is EXOthermic.
ENDOTHERMIC
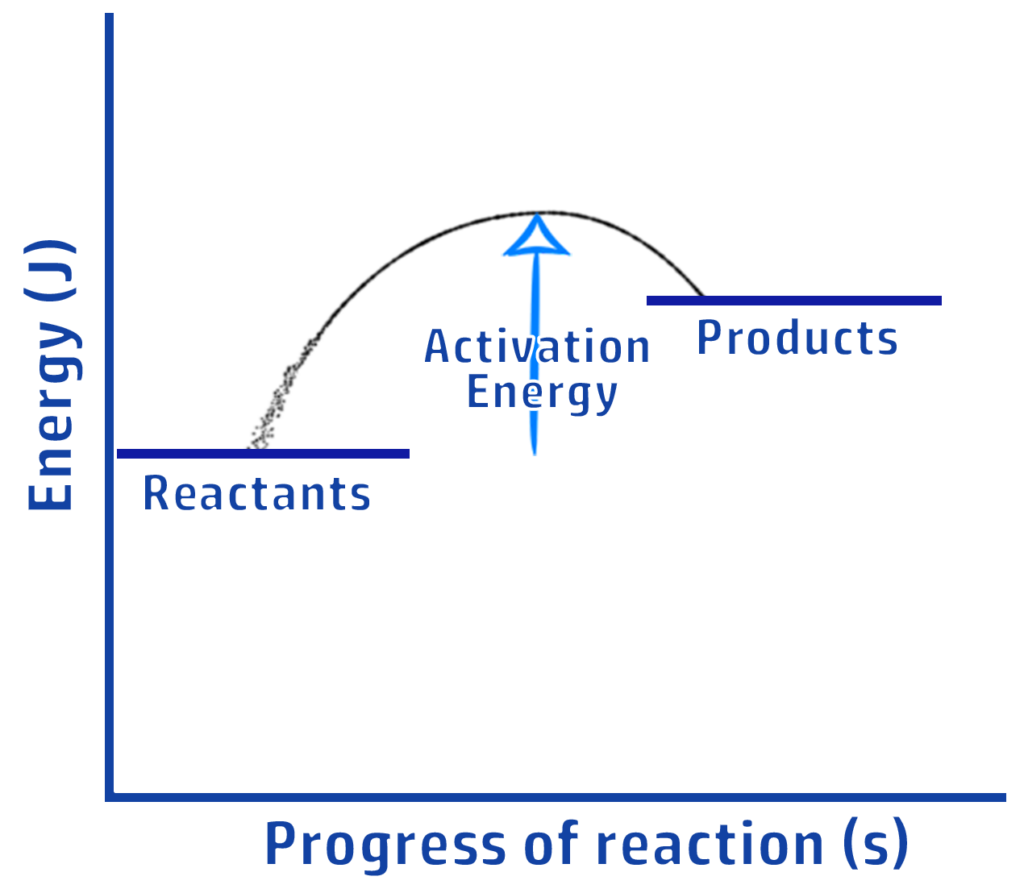
Clue: More people have entered the shop (products have more energy) so it is ENDOthermic.
CH204: Describe bond breaking and bond forming
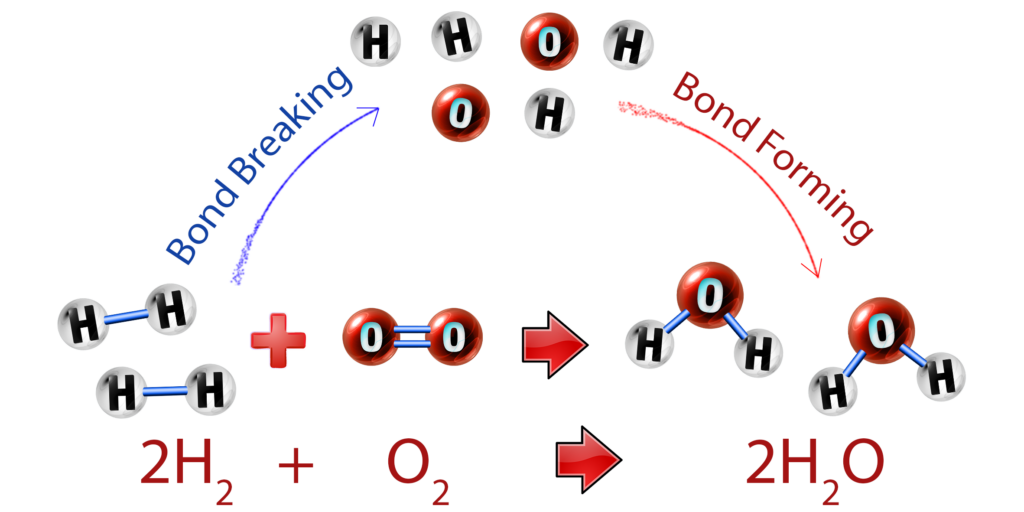
There are two key things to remember that occur in every chemical reaction:
- Bond breaking is endothermic – heat energy is taken in to break the bonds.
- Bond forming is exothermic – heat energy is given out to the surroundings when new bonds form.
CH205: Explain what makes a reaction exothermic overall
For a reaction to be exothermic overall, more energy must be given out to the surroundings (when bonds form) than taken in (when bonds are broken).

For a reaction to be endothermic overall, more heat energy must be taken in from the surroundings (when bonds are broken) than given out (when new bonds are formed).
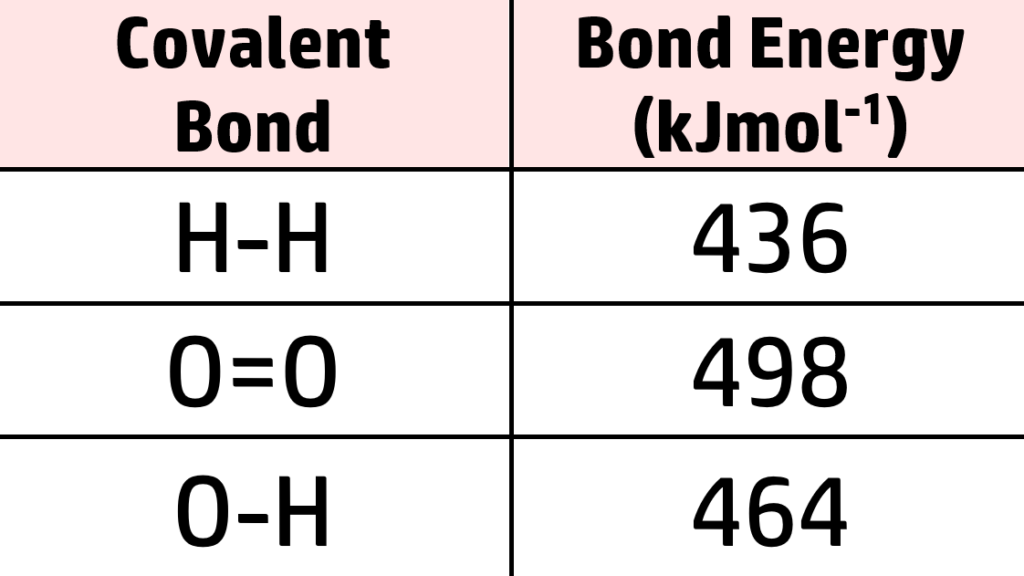
CH206: Calculating Energy Changes (H)
In a chemical reaction, you need a certain amount of energy to break bonds (endothermic) and a certain amount of energy is given out to form new bonds (exothermic). You can, therefore, use a reference table (such as the one on the right) to work out if a reaction is exothermic or endothermic.
Example: Calculate the energy change in the reaction between hydrogen and oxygen to form water:
2H2 + O2 → 2H2O
Step 1: Work out the energy needed to break the bonds in H2 and O2:
2H2 + O2 →
We have 2 hydrogen molecules and one oxygen molecule.
From the table above, you can see that:
- 436kJmol-1of energy is needed to break the bonds in each hydrogen molecule. We have 2 molecules of hydrogen, so 2 x 436 = 872kJmol-1
- We have one oxygen molecule, so 498kJmol-1 is needed.
Therefore, the total energy to break the bonds is 872 + 498 = 1370kJmol-1 is needed to break all bonds.
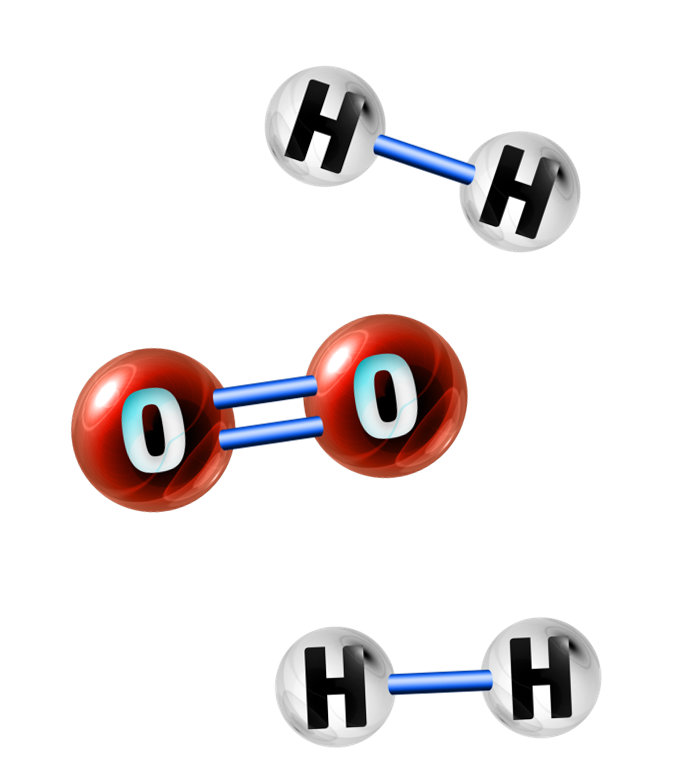
Step 2: Work out the energy needed to form the bonds in H2O:
→ 2H2O
Each water molecule has 2 hydrogens attached to one oxygen. Therefore, each H2O has two O=H bonds.
- 464kJmol-1of energy is needed to break the O- H bond.
- Each water has 2xO-H bonds, so each water = 928kJmol-1.
We have 2 water molecules, so 928x2 = 1856kJmol-1 needed to break all bonds in 2H2O.

Step 3: Work out the energy change, which is bond breaking (reactants) – bond forming (products):
- 1370kJmol-1 - 1856kJmol-1 = -486kJmol-1
- Make sure to put the sign (+ or -) – this is worth one mark!
CH207: Describe what a catalyst is
Catalysts are chemicals that:
- Speed up the rate of a chemical reaction,
- Remain chemically unchanged at the end of a reaction,
- Keep the same mass during the reaction.
This means that catalysts can be reused. One of the biggest examples of a use for a catalyst is in catalytic converters in cars.

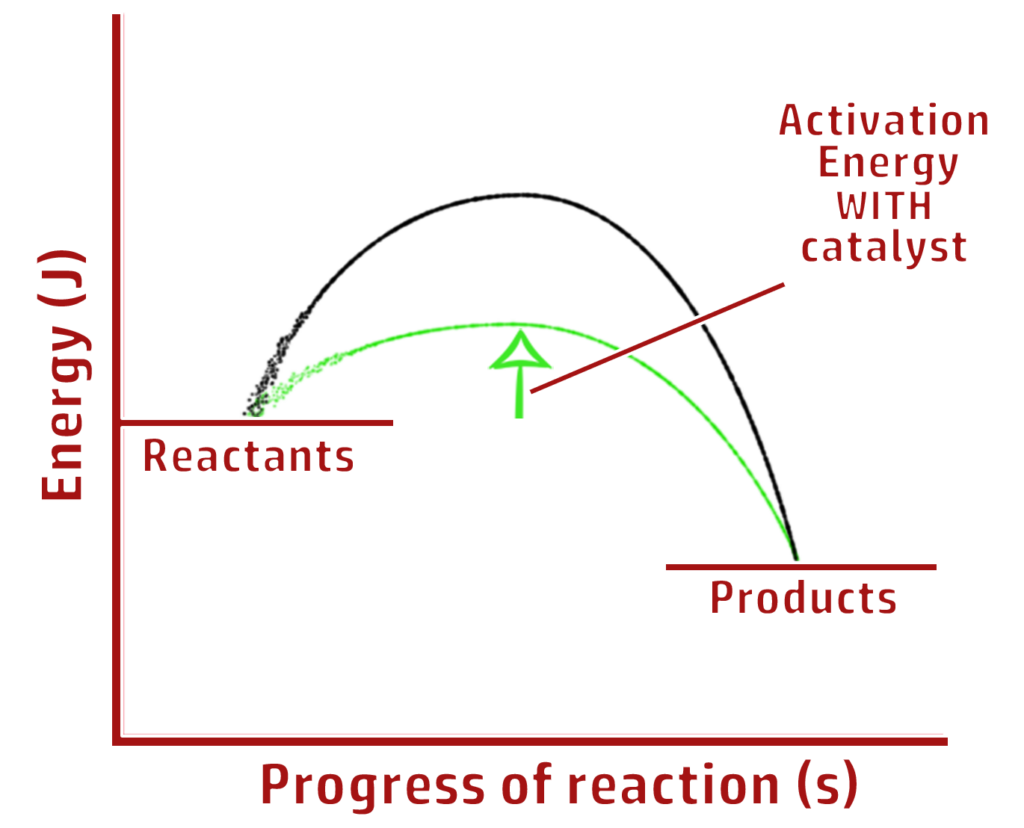
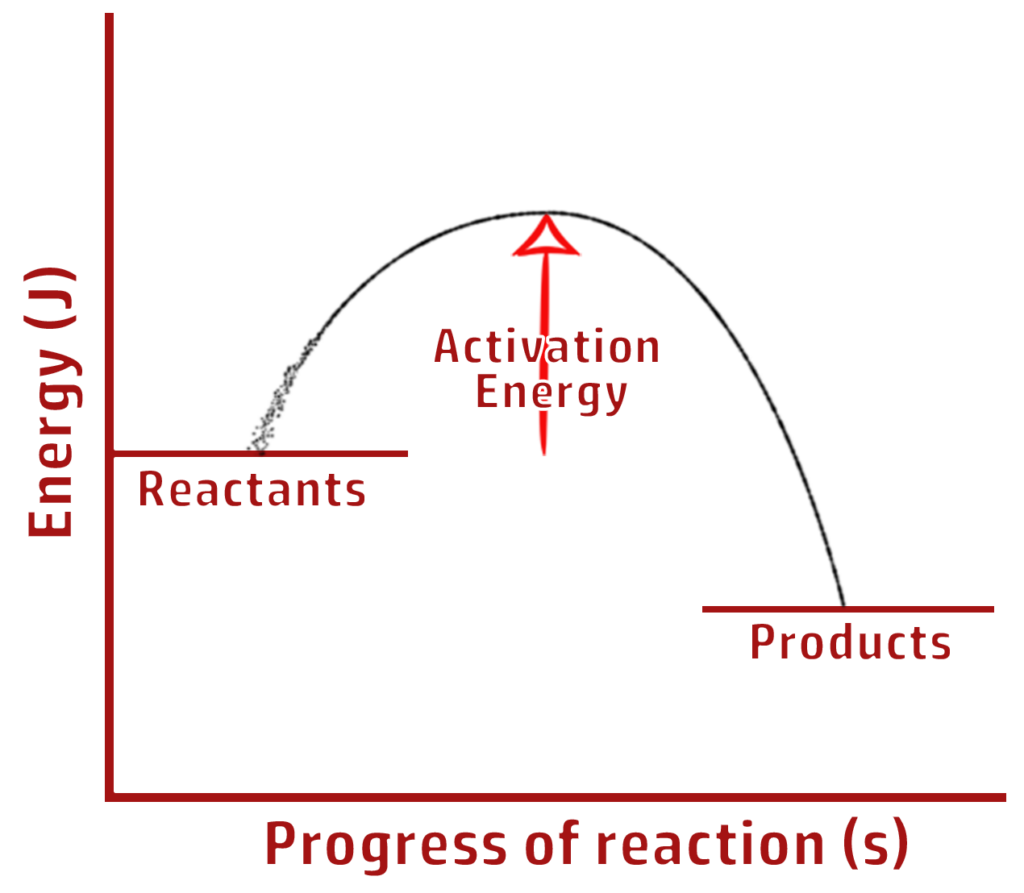
CH208: Describe what activation energy is
Activation energy is the energy required to break the bonds that start a reaction.
- If a collision occurs without enough (activation) energy, the bonds will not be broken, and the reaction cannot occur.
- If the collision has enough (activation) energy, bonds can be broken and therefore the reaction can occur.
CH209: Compare catalysts to enzymes
Enzymes, which are biological catalysts, are used to speed up reactions such as alcoholic drink production, respiration, photosynthesis, and protein synthesis.
- Enzymes contain an active site, where the reactant molecule (the substrate) bonds in a lock and key type mechanism.
- Enzymes can be denatured if the pH or temperature changes.
CH210: Explain the effect of catalysts on activation energy
Catalysts work by lowering the activation energy (by providing a different route for a reaction).
- This means that more collisions have enough energy to break the bonds to start a chemical reaction.
- Therefore, more collisions are successful – speeding up the chemical reaction.