METALS AND EQUILIBRIA

A GCSE revision page explaining metals and equilibria including reactivity of metals, extraction, displacement reactions, and dynamic equilibrium concepts with practical examples and explanations of how systems respond to changes.
CH142: Linking observations and reactivity
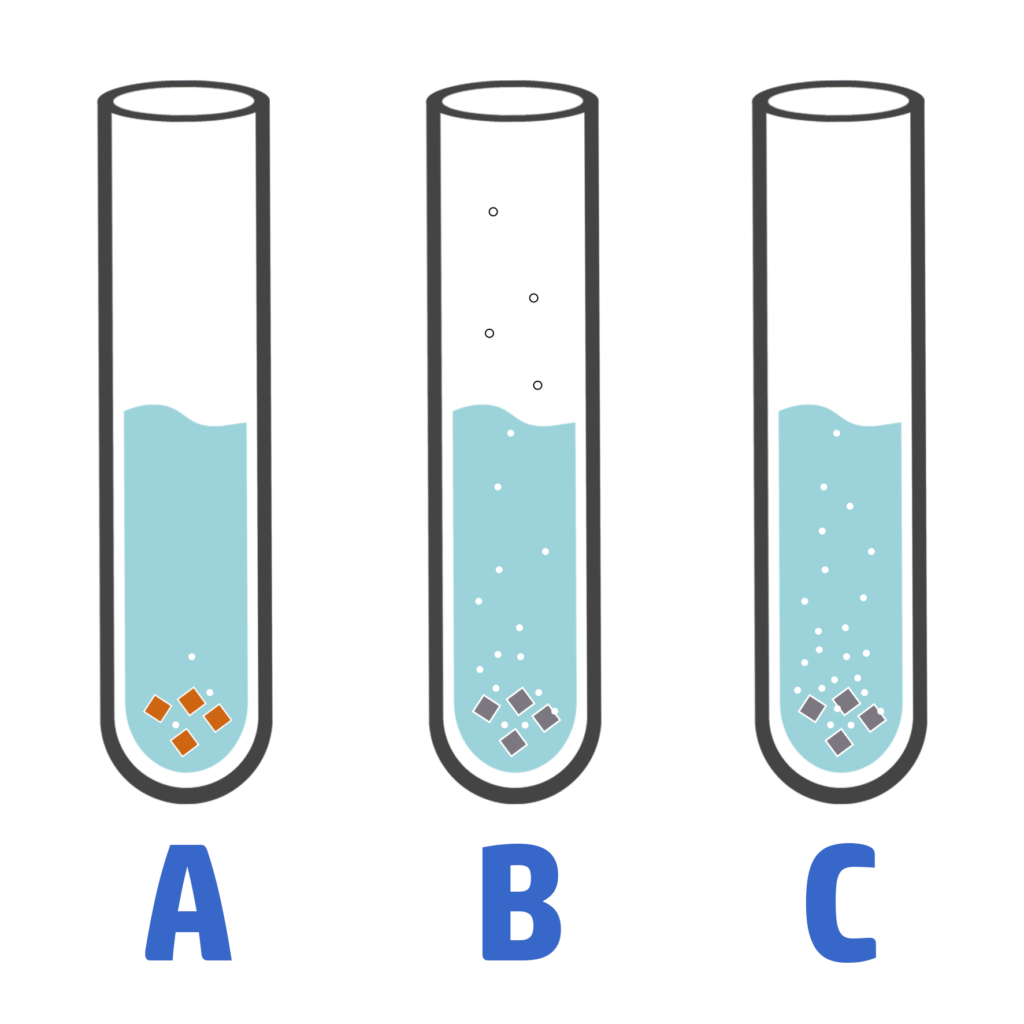
When you react a metal with water and different acids, you can use the observations to work out how reactive they are:
- The more bubbles / fizzing / effervescence there is, the more reactive the metal.
- On the right, you can see that C is the most reactive metal because it produces the most bubbles.
- A is the least reactive as it has the least bubbles.
You can also look at temperature changes. The bigger the temperature change, the more reactive the metal.
CH143: Displacement reactions and REDOX (H)
When you add a metal to a solution containing a metal, you can predict whether a displacement reaction will occur or not (where a more reactive metal swaps places with a less reactive metal in a compound).
The more reactive metal wants to be part of the compound (see CH142) – leaving the least reactive as the stand-alone metal.
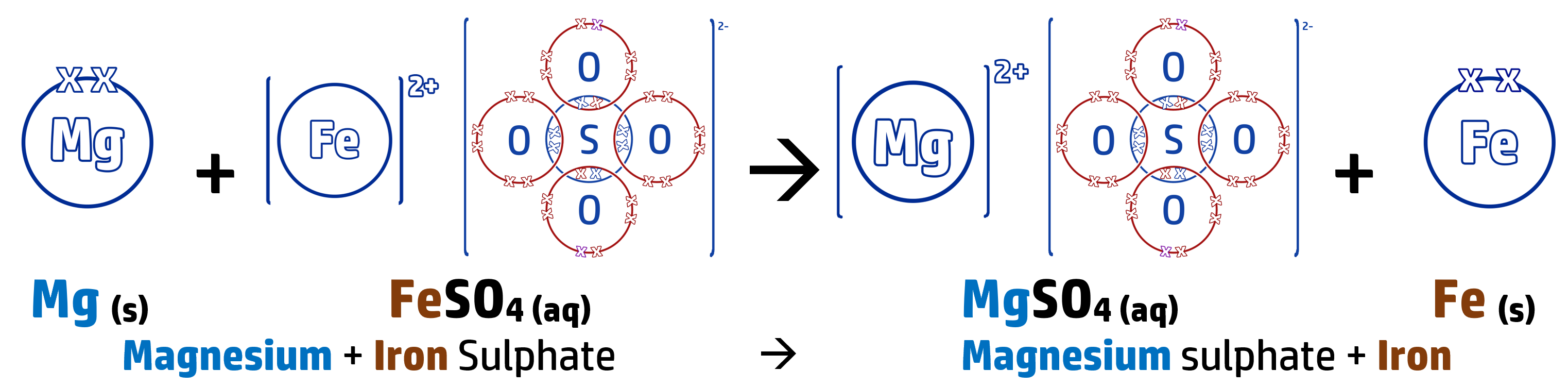
In the above reaction, magnesium is more reactive than iron so swaps places with it.
This is an example of a REDOX reaction – where oxidation and reduction are both occurring during the reaction:
Magnesium turns from an atom into an ion by losing two electrons – this means that the magnesium is oxidised (OIL – see CH140).
Mg → Mg2+ + 2e-
The iron ion, which is displaced, gains two electrons to turn back into an iron atom. The iron is therefore reduced (RIG – see CH140).
Fe2+ + 2e- → Fe
CH144: Investigate the reactivity of metals
You can work out the reactivity series for metals by seeing how they react with cold water, steam, and acids. The results below allow you to put the metals in order of reactivity:
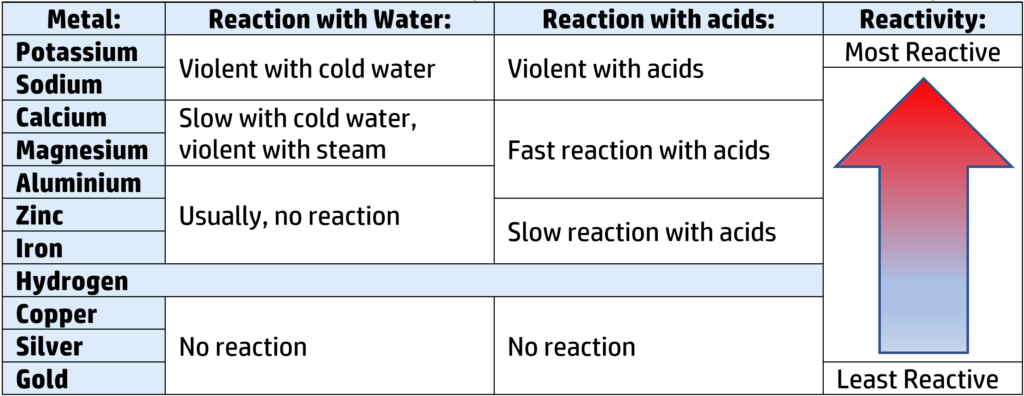
Reactions of metals with water:
If a metal reacts with water, if will always form a metal hydroxide and hydrogen:
Potassium + water → Potassium hydroxide + hydrogen
Reactions of metals with steam:
If a metal reacts with steam, if will usually form a metal oxide and hydrogen:
Calcium + water → Calcium oxide + hydrogen
Reactions of metals with acids:
If a metal reacts with an acid, if will usually form a salt and hydrogen (see CH116):
Calcium + nitric acid → Calcium nitrate + hydrogen
CH145: Linking electronic configuration to reactivity
For a metal to oxidise, it needs to lose its electrons / react with oxygen.
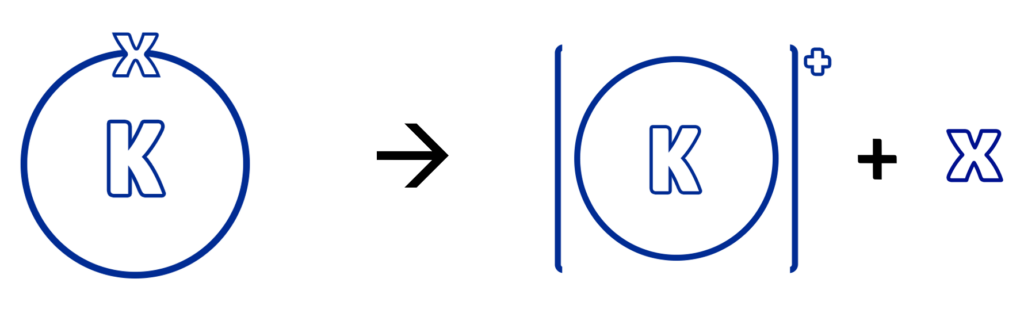
The more reactive a metal is, the quicker it is to lose its electrons/react with oxygen.
Therefore:
- If a metal is highly reactive, such as potassium, it will oxidise/lose its electrons very quickly.
- If a metal is less reactive, such as iron, it will oxidise slowly.
- If a metal is unreactive, such as gold, it will not oxidise at all.
CH146: Describe what an ore is

An ore is a rock that contains enough metal to make it financially worthwhile to extract. If it isn’t going to give a profit to extract it – it is not an ore!
Most metals are found in ores, such as bauxite – aluminium oxide, Al2O3, and need to be extracted by either heating with carbon or electrolysis (see CH149)
Some metals, such as gold and silver, are found uncombined – these are the metals that don’t react – so are not found in ores.
CH147: Describe Oxidation and Reduction in terms of oxygen
There are two definitions of oxidation and reduction – one involving electrons (see CH140) and one involving oxygen, which states that:
- Oxidation is the addition of oxygen.
- Reduction is the removal of oxygen.
For example: The reduction of iron oxide, FeO, using carbon:
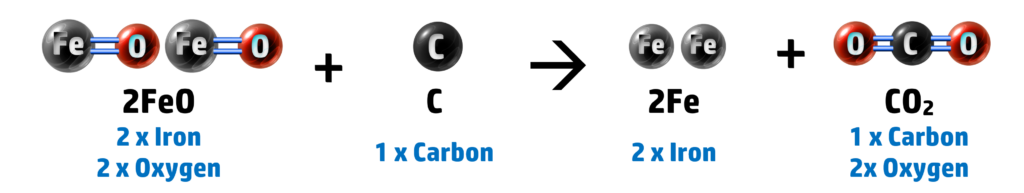
If we look at the two atoms that are not oxygen, we have iron and oxygen:
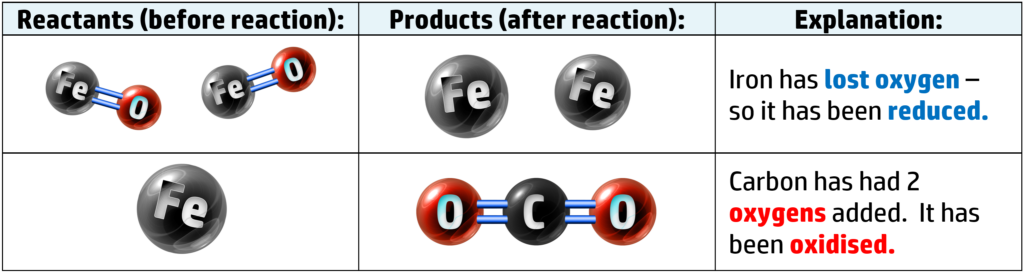
When metals are oxidised, they corrode. When iron rusts, it isn’t just reacting with oxygen, but water as well.
CH148: Link metal extraction to oxidation and reduction
Regardless of which type of metal extraction you look at (either electrolysis or heating with carbon – see CH149), reduction is always occurring.
Reduction is the removal of oxygen and the gain of electrons.
1. Electrolysis: 2Al2O3 → 4Al + 3O2
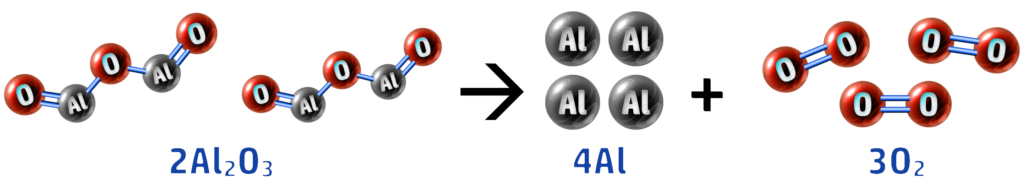
Here you can see that our aluminium oxide has lost oxygen (forming aluminium and oxygen) - therefore aluminium oxide is reduced.
2. Heating with Carbon: 2FeO + C → 2Fe + CO2

Here you can see that our iron oxide has lost oxygen (forming iron and carbon dioxide) - therefore iron oxide is reduced. Carbon has also been oxidised to form carbon dioxide (see CH147).
CH149: Explain how to extract metals based on cost and reactivity

There are three ways of removing metals from the ground:
Electrolysis is used to extract highly reactive elements from their ore. These elements are more reactive than carbon.
Heating with carbon is used to extract slightly reactive metals from their ore. These elements are less reactive than carbon.
Unreactive metals, such as gold and silver, can just be removed by digging.
CH150: Evaluate Bioleaching and Phytoextraction (H)
There are two other methods of extracting metals which are used to extract rocks with small amounts of metals in – called low grade ores: Bioleaching and Phytoextraction.
Bioleaching:
- Bioleaching uses bacteria to break down low-grade ores into a solution called a leachate.
- This solution is acidic and contains the metal ions you want.
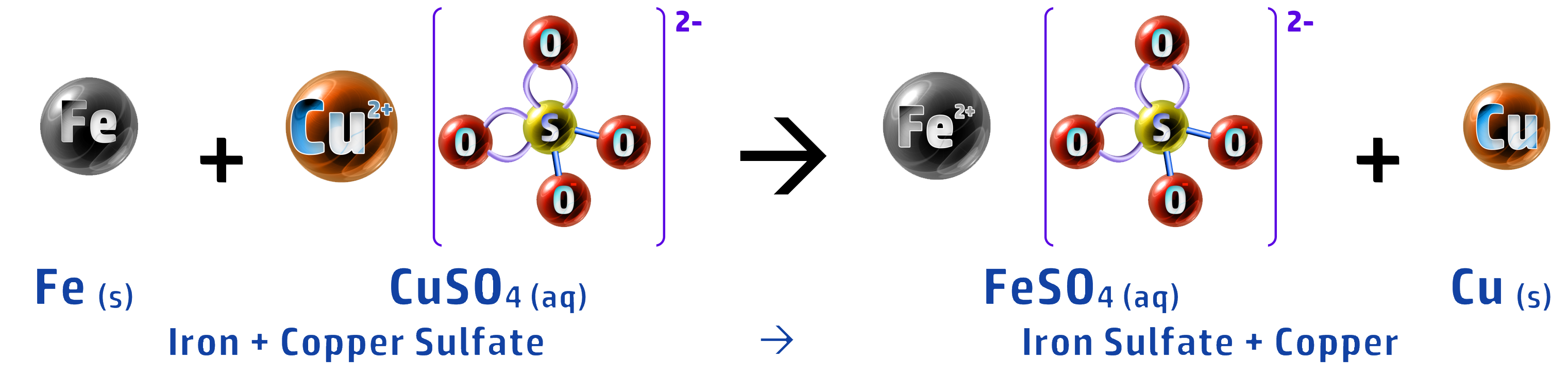
- An example of this is collecting copper, from a copper sulfate leachate, using scrap iron.
- Iron is more reactive than copper so displaces it:
| Advantages: | Disadvantages: |
|---|---|
| Does not require higher temperatures. No harmful gases Less damage to landscape than mining Conserves supplies of higher-grade ores | Very slow. Toxic substances and sulphuric acid can be produced – damaging the environment |
Phytoextraction
- Plants absorb mineral ions from the soil.
- The metal ions become concentrated in the plant cells.
- The plants are burnt, leaving behind the metal in the ash.
| Advantages: | Disadvantages: |
|---|---|
| Can extract metals from contaminated soil. No harmful gases Less damage to landscape than mining Conserves supplies of higher-grade ores | Very slow. Can only grow in certain climates |
CH151: Describe the advantages and disadvantages of Recycling
Advantages of recycling:
- It conserves Earth’s natural resources – meaning we won’t run out as fast!
- Less mining of ores is needed. This is good because:
- It doesn’t damage the landscape.
- It doesn’t create noise/dust pollution.
- It can take less energy to recycle than to extract from the ore.
- Metals don’t end up wasted in landfill sites.
Disadvantages of recycling:
- It takes time for people to sort through all of the different plastics / metals.
- It costs money to employ people to sort the plastics / metals.
- Some materials cannot be recycled.

CH152: Life Cycle Assessments
A lifestyle assessment looks at the total environmental cost of a product by looking at each stage of the life of a product:
| Stage: | Description: |
|---|---|
| Choice of material | When metals are extracted from their ores, it needs a lot of energy and can produce a lot of pollution. Raw materials can come from crude oil, which is non-renewable. Crude oil also gives out greenhouse gases when combustion occurs. |
| Manufacture of product | Manufacturing needs a lot of energy. It can cause a lot of pollution. Waste products need disposing of safely – some can be recycled. The water used in lots of manufacturing needs to be safe/unpolluted when put back into the environment |
| Use of product | The products can themselves be harmful – such as: Toxic fumes from paint, Toxic gases from combustion and Fertilisers draining into lakes/rivers causing eutrophication |
| Disposal of product | Lots of products are disposed of in landfills, which takes up space and can pollute the land/water. Products can also be burnt – which can give off toxic gases or greenhouse gases |
Each of these factors need to be considered when manufacturing a product. If there are multiple ways of producing a product, the one that has the least environmental cost will be chosen.
CH153: Evaluating Life Cycle Assessments
Example: A decision needs to be made about whether to produce a cabinet from two different sources:
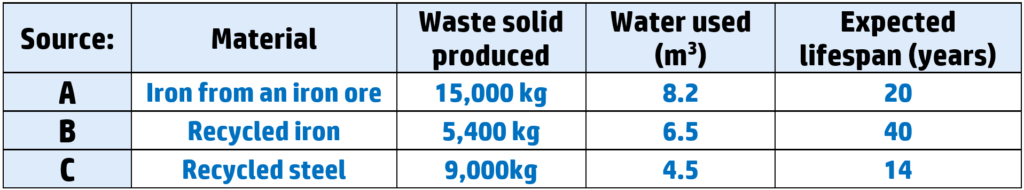
You would not choose A because the iron comes from an ore – which takes more energy to extract than recycling. It also produces the most waste and uses more water.
You would choose B because it is recycled, which uses less energy, and produces the least waste. It also will last the longest and therefore has the least environmental cost.
CH154: What are Reversible Reactions?
A reversible reaction is any reaction that can go in both the forward direction and backwards direction.
- It has a different symbol: ‘⇌’ instead of '→'
- This can be at the same time, or separately.
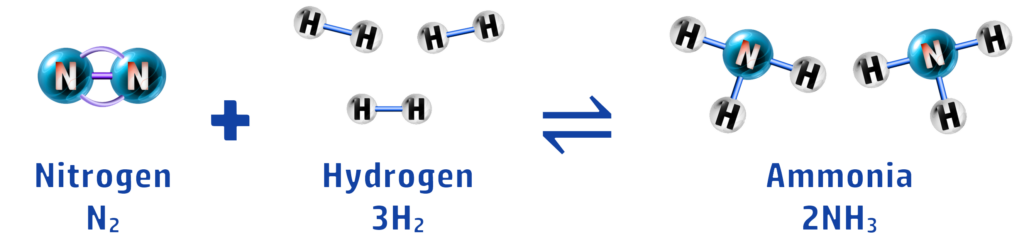
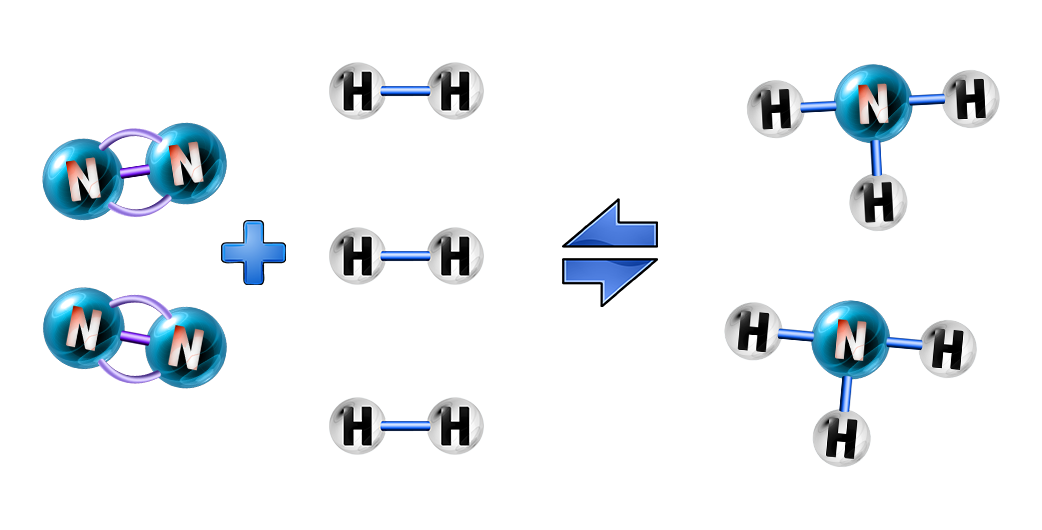
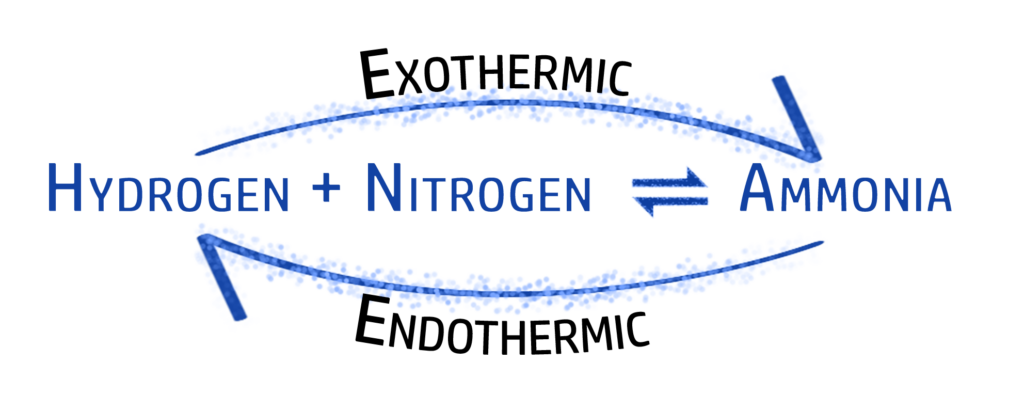
In this reaction, you can see that nitrogen and hydrogen react to form ammonia, and ammonia decomposes to form nitrogen and ammonia. To make it easier to write, we use the ⇌ symbol.
CH155: Explaining Dynamic Equilibrium
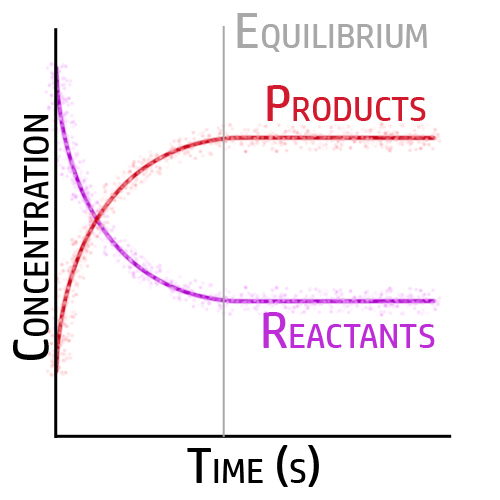
When the forward reaction and backward reaction are occurring at the same rate, and the concentrations do not change – it is at equilibrium.
When the forward and backwards reactions are occurring at the same time and same rate, it is at dynamic equilibrium.
This means that:
- The forward reaction and backward reaction are occurring at the same rate/speed.
- The concentration does not change.
- The forward reaction is occurring.
- The backwards reaction is occurring.
This doesn’t mean that there is 50% of products and 50% of reactants though…
- There may be a higher percentage of products, 80% for example, but it will stay at 80% whilst at equilibrium.
- Similarly, if there is 60% of reactants and it is at dynamic equilibrium, it will stay at 60%.
Remember, for something to be at dynamic equilibrium, it must be in a closed system – this means that no products and reactants should be allowed to escape!
CH156: How is ammonia produced?
Ammonia is a chemical that is used in the manufacture of fertilisers. Nitrogen (from air) and hydrogen (from natural gas)are reacted together, using high temperature and pressure (see CH157), to form ammonia. This is a reversible reaction (see CH154):

CH157: Recall the conditions for the Haber Process
| Temperature: 450oC | Pressure: 200atm | Catalyst: Iron |
|---|---|---|
| 450oC is the temperature used in the Haber Process. It is a compromise between yield (amount made) and rate of production. (See CH158) | The Haber process is carried out at 200atm. This is a compromise between the cost of the equipment and yield of ammonia (See CH158) | Iron is the catalyst used in the Haber Process. Catalysts speed up the rate of reaction, increasing the yield, but does not change the position of equilibrium (CH158). |
CH158: Explain the effect of temperature and pressure on the Haber Process
Temperature
If you increase the temperature, you will always get a higher yield for the endothermic reaction.
In the Haber process, the backwards reaction is endothermic. This means that by increasing the temperature, you will have a lower yield of ammonia (and more nitrogen/hydrogen).
450oC is a compromise between yield and rate.
Pressure / Concentration
If you increase the pressure / concentration, the particles become closer – meaning there are more collisions.
This favours the side with the least particles – the Haber Process has a ratio of 4:2 in favour of ammonia - giving a higher yield of ammonia.
Increasing the pressure is very expensive, however.
200atm is a compromise between yield and cost.