STATES OF MATTER AND SEPARATION TECHNIQUES
CH89: Describe the arrangement and movement of solids, liquids and gases
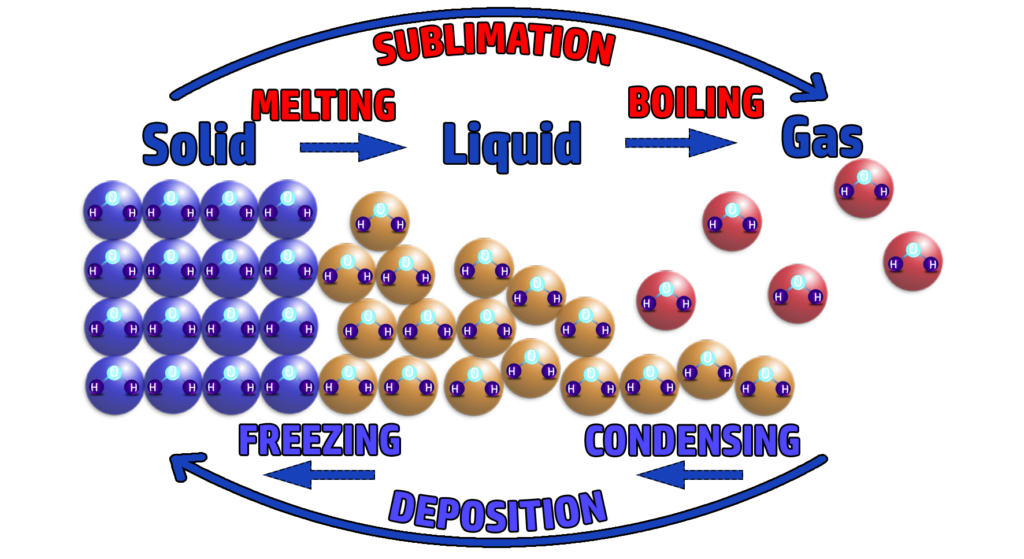
SOLIDS
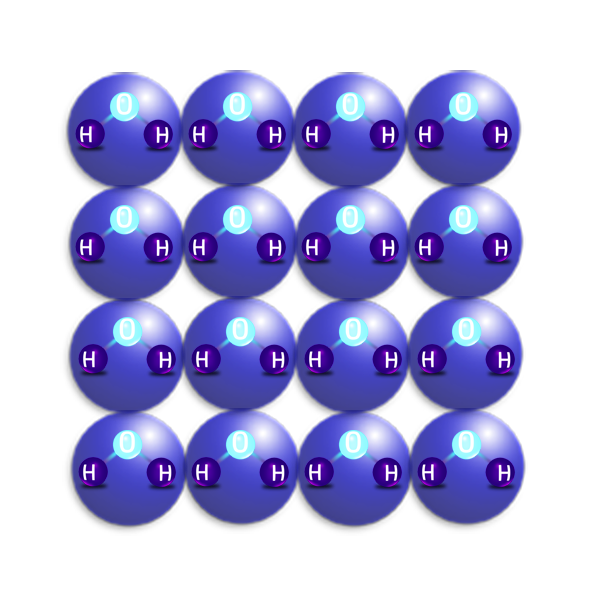
- Solids have a regular arrangement (in rows) and all the particles are touching.
- They don’t move – but vibrate about a fixed position.
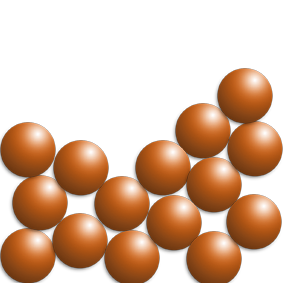
LIQUIDS
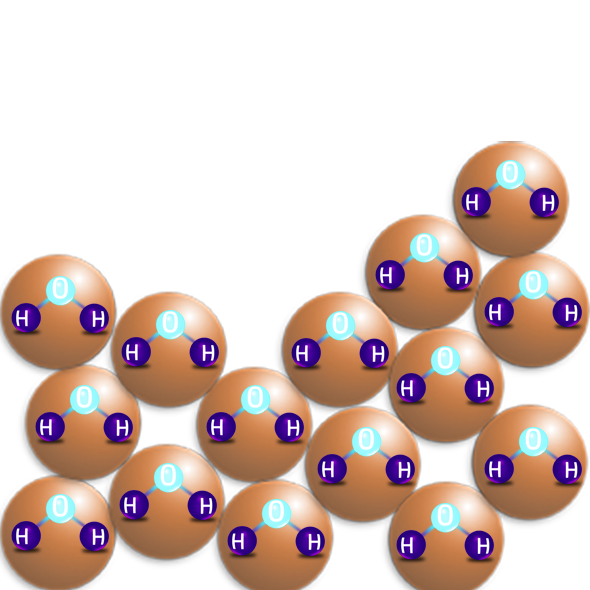
- Liquids have a random arrangement, but the particles are all still touching.
- The particles are free to move about each other – they flow.
GASES
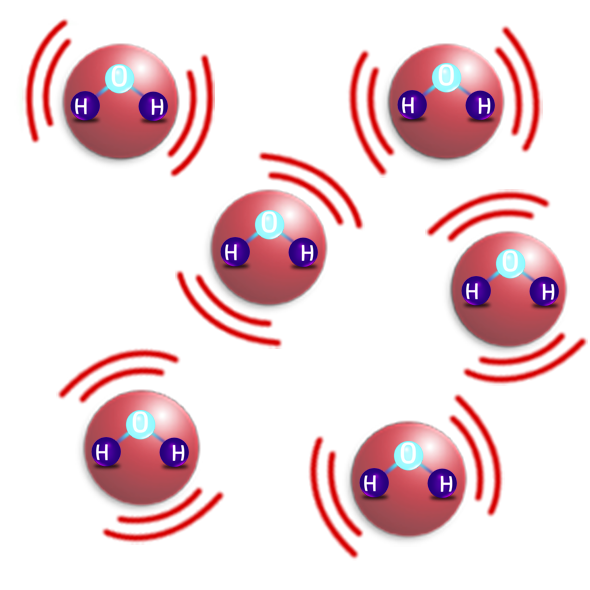
- Gas particles are in a random arrangement and not touching.
- They are moving fast in all directions.
CH90: Identify the state changes
CH91: Explain what state change graphs show us
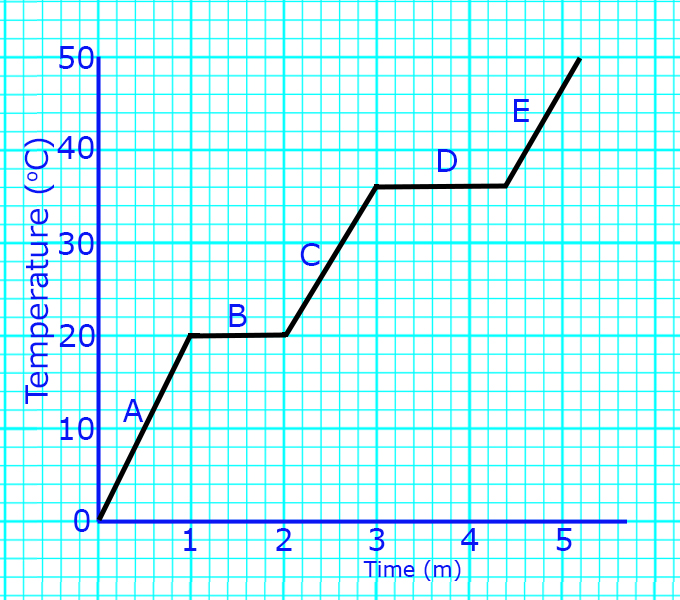
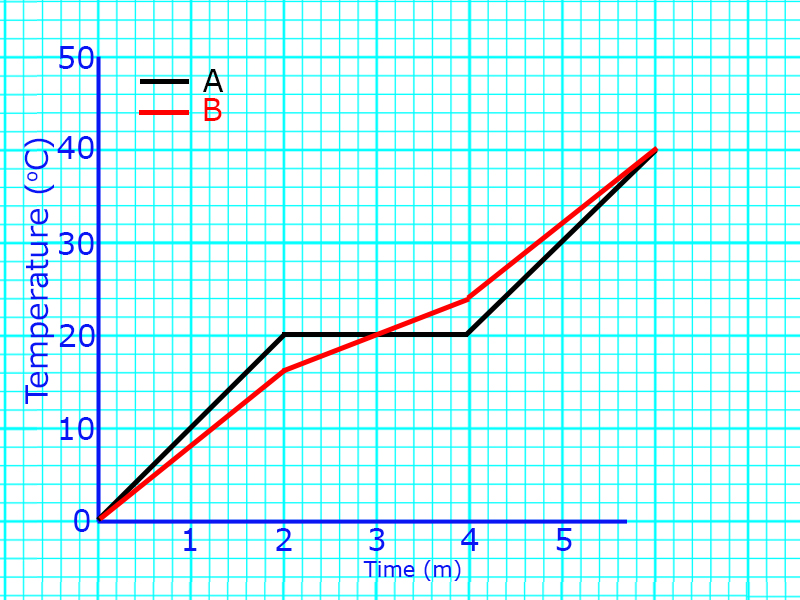
A
At A, the solid is being heated.
The particles are vibrating about a fixed position, but still in a regular arrangement.
B
At B, melting occurs. The temperature does not rise because the energy is being used to weaken the intermolecular forces.
The particles go from a regular pattern to a random pattern and start to flow.
C
At C, the liquid is being heated.
The particles are now free to flow but are still touching. As they are heated, they will move faster.
D
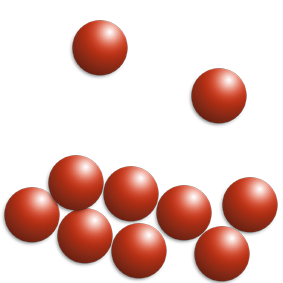
At D, boiling is occurring.
The temperature does not rise because the energy is being used to break the intermolecular forces.
The particles go from touching to being far apart and moving fast in all directions.
E
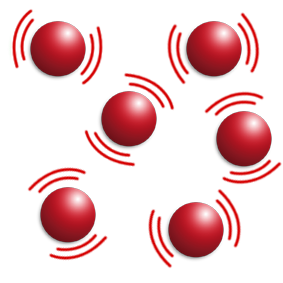
At E, the gas is being heated.
The particles are now not touching and moving fast in all directions.
CH92: Predict the state of a substance from melting points / boiling points
If you are asked to predict whether a substance is a solid, liquid or gas - Draw a diagram to show you the melting and boiling points.
Label the melting point and boiling point on the graph. For example, if the temperature given in the question is:
- Less than the melting point = SOLID
- Between melting and boiling point = LIQUID
- More than the boiling point = GAS
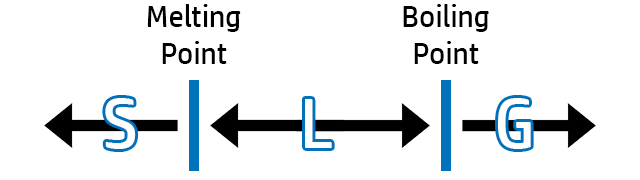
Example: If a substance has a melting point of 35oC and a boiling point of 250oC, what is the state at 60oC?
Answer: 60oC is ‘in between’ the melting point (35oC) and boiling point (250oC), so it will be a LIQUID.
CH93: Describe what a pure substance is
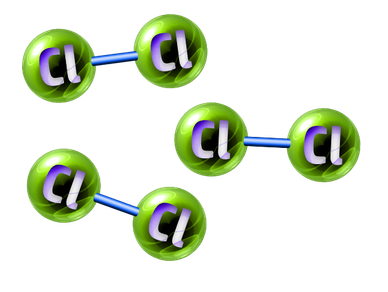
Pure Elements:
An element is any substance that contains one type of atom.
A pure element is any substance that only contains one type of element.
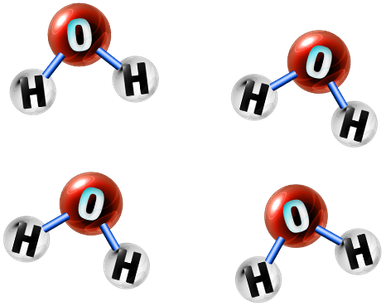
Pure Compounds:
A compound is any substance that contains more than one type of element bonded.
A pure compound is any substance that only contains one type of compound.
CH94: Explain how to identify a mixture using a state change graph
Mixtures
A mixture is a substance that contains more than one type of substance (compound or element) that is not bonded.
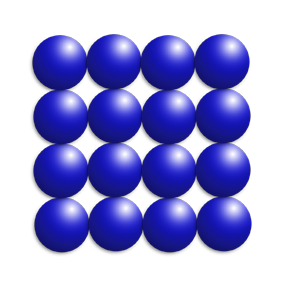
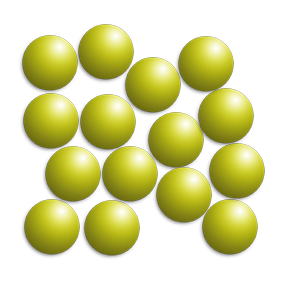
You can find out if you have a mixture by looking at a melting point graph.
If there is one melting point (a flat line) – it is pure, as only one substance is melting.
If there is more than one melting point (a diagonal line), it is a mixture because you have more than one substance melting at different times.
On the right, substance A is pure and substance B is a mixture.
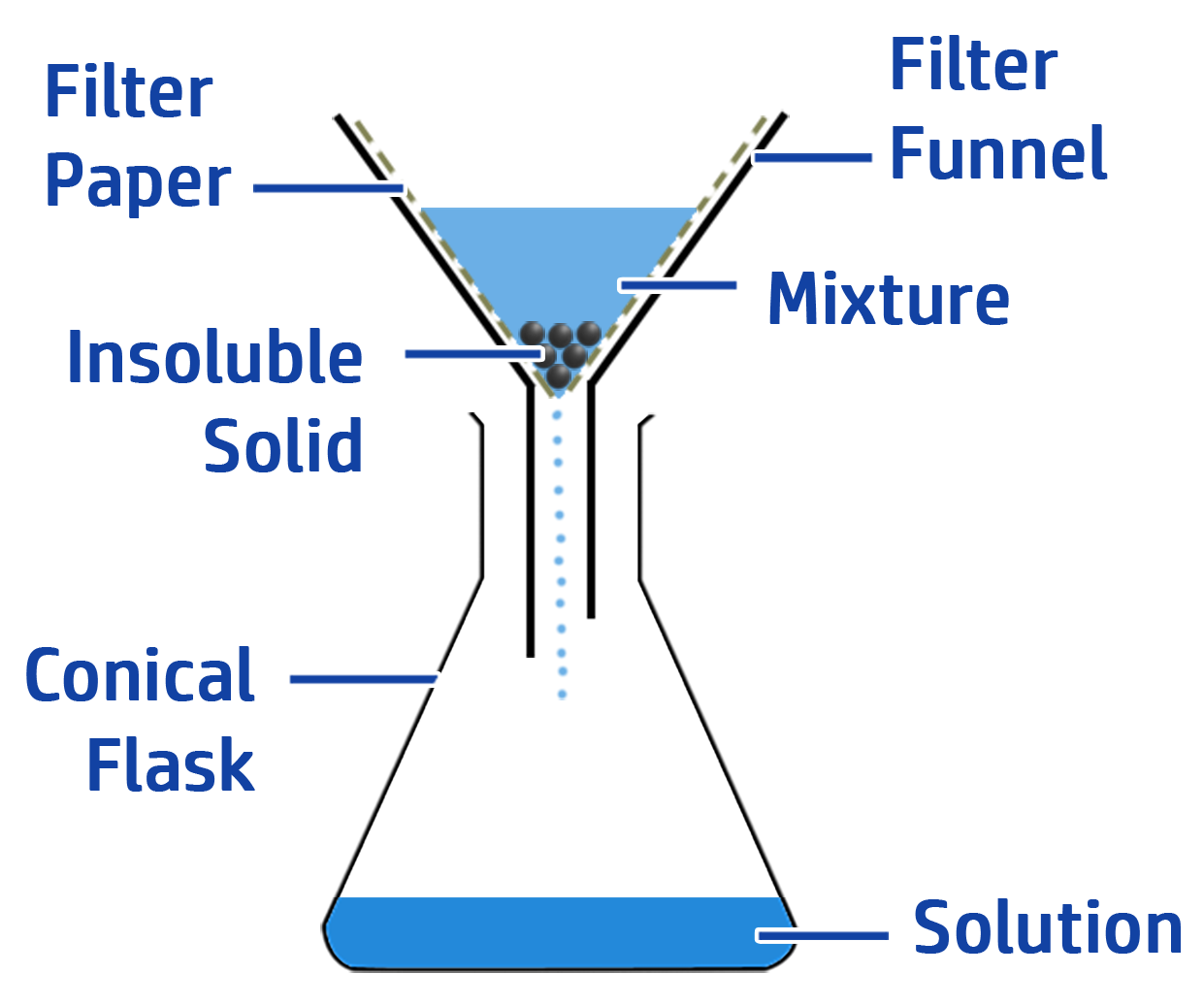
CH95: Describe how to carry out filtration
An insoluble solid is a solid that will not dissolve – such as rock/sand/copper oxide.
To separate an insoluble solid from a mixture:
- Step 1: Add water to dissolve anything that is soluble. The insoluble solid will remain as a solid.
- Step 2: Add the mixture to a filter funnel with filter paper in it.
The soluble solids and water will move through the filter paper (because their particles are small enough to fit through the holes in the filter paper). The insoluble particles will be too big, so will remain in the filter paper.
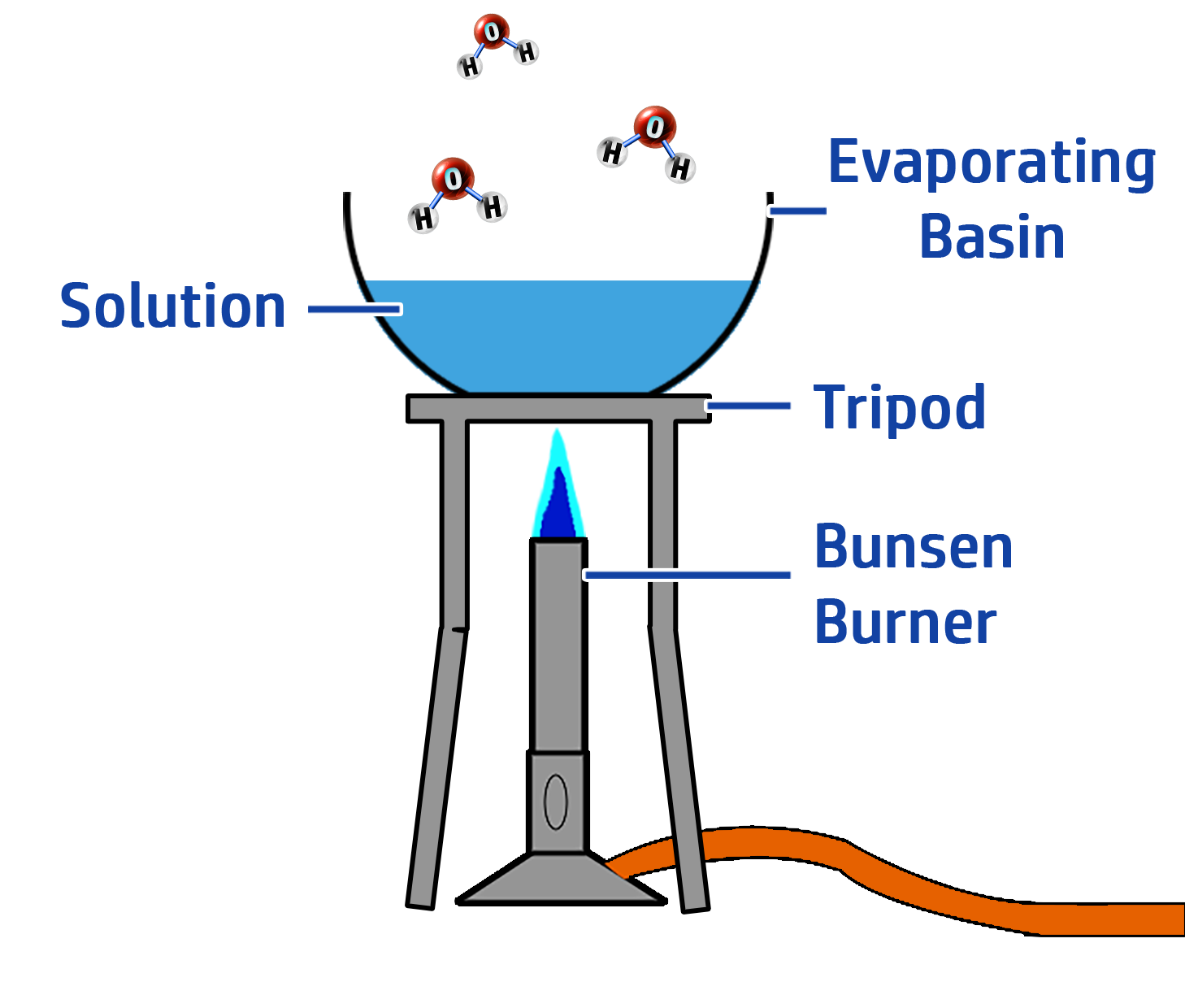
CH96: Describe how to carry out crystallisation
A soluble solid is a solid that will dissolve when added water, or other solvents. An example of a soluble salt is table salt (sodium chloride)
To separate a soluble solid from a mixture:
- Step 1: Heat the solution gently using a Bunsen Burner (see right)
- Step 2: Evaporate half of the solution
- Step 3: Leave the solution to cool.
- Step 4: Leave the crystals to dry on a windowsill.
CH97: Describe how to separate soluble and insoluble solids
If you have a mixture of a soluble and insoluble solid, you need to carry out filtration and crystallisation.
- Step 1: Dissolve the mixture with water.
- Step 2: Add the mixture to a filter funnel and filter paper. This will separate the insoluble solid from the rest of the mixture.
- Step 3: Add the solution (filtrate) to an evaporating basin and heat the solution until half of the solution has evaporated.
- Step 4: Leave to cool/evaporate – leaving crystals behind.
CH98: Describe how to prepare a chromatogram
To prepare a chromatogram, you need to:
- Put a line in pencil (which is insoluble) on the bottom of the chromatogram.
- Add your sample(s) to the crosses (which should also be pencil).
- Once done, add the chromatogram to water making sure the ink does not touch the water. (If it does, the ink will dissolve and not move up the paper!)
- Wait until the water is close to the top of the chromatogram and remove. Mark the water line with a pencil.
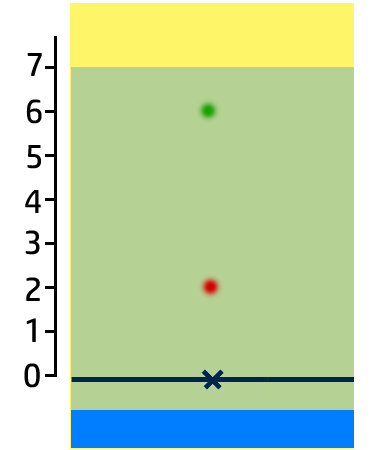
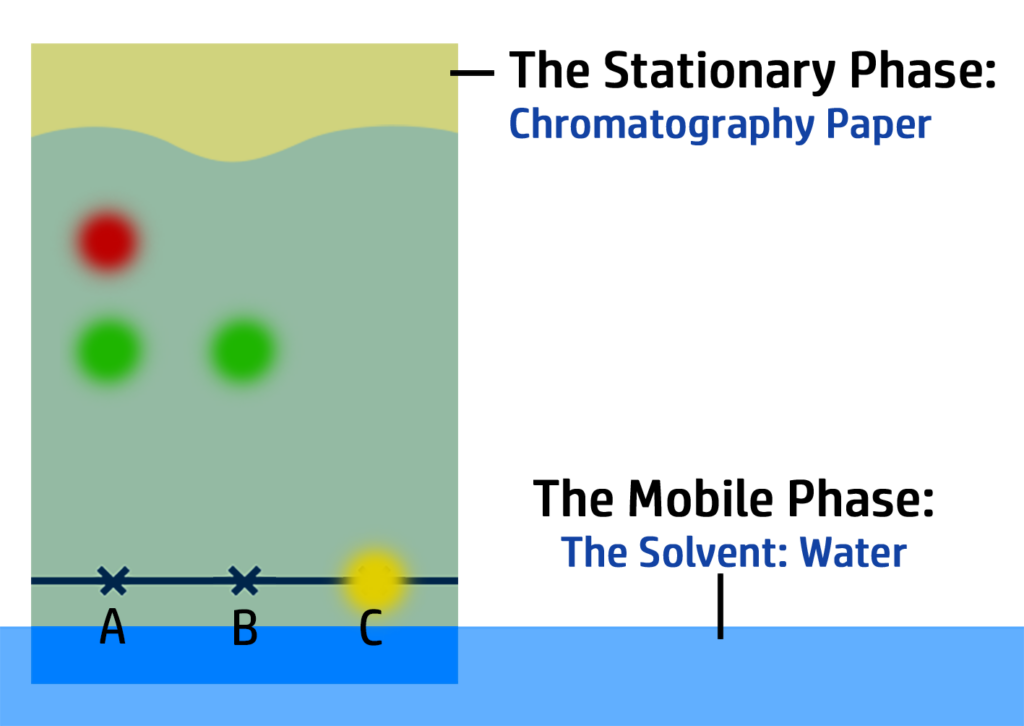
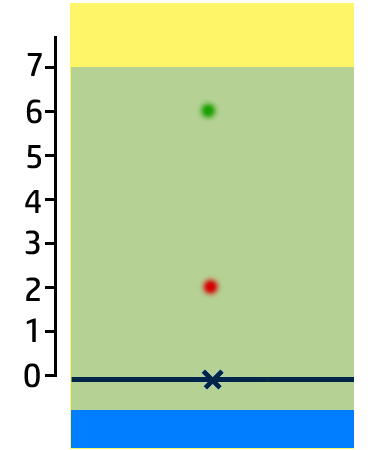
CH100: Analyse the results of a chromatogram
In the chromatogram on the right, you can see that:
- Substance A contains one colour – blue – so it is pure.
- Substance B has two colours – red and green – so it is a mixture.
- Substance C is insoluble in water.
You can also work out how soluble the colours are:
- The most soluble colour in this chromatogram is green. It has moved the furthest, so is the most soluble.
- Red is the least soluble (of the ones that have dissolved!)
- Yellow is insoluble.
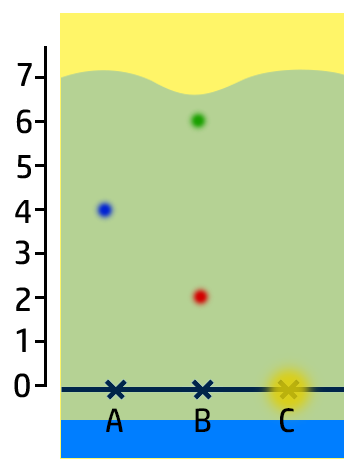
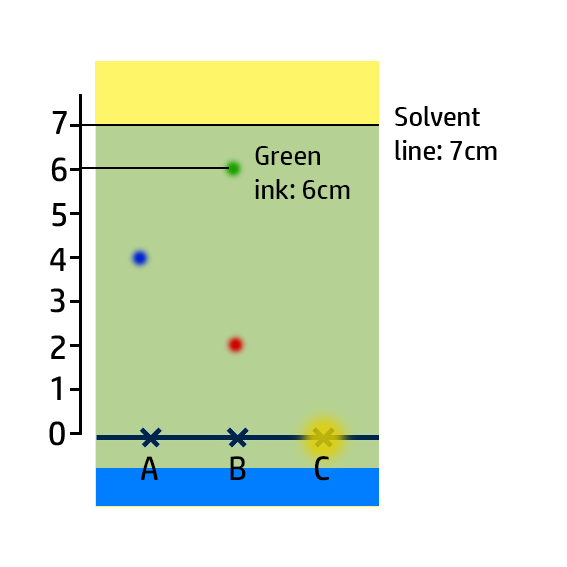
CH101: Calculate Retention Factor, Rf
The Retention Factor, Rf, is a measure of how soluble an ink is.

To calculate the retention factor, dissolve the distance the ink moves by the distance the water moves.
Example:
- For the green ink, it has moved 6cm, whilst the water has moved 7cm.
- Therefore 6cm / 7cm = 0.86.
Key Check: The retention factor will always be between 0 and 1!!
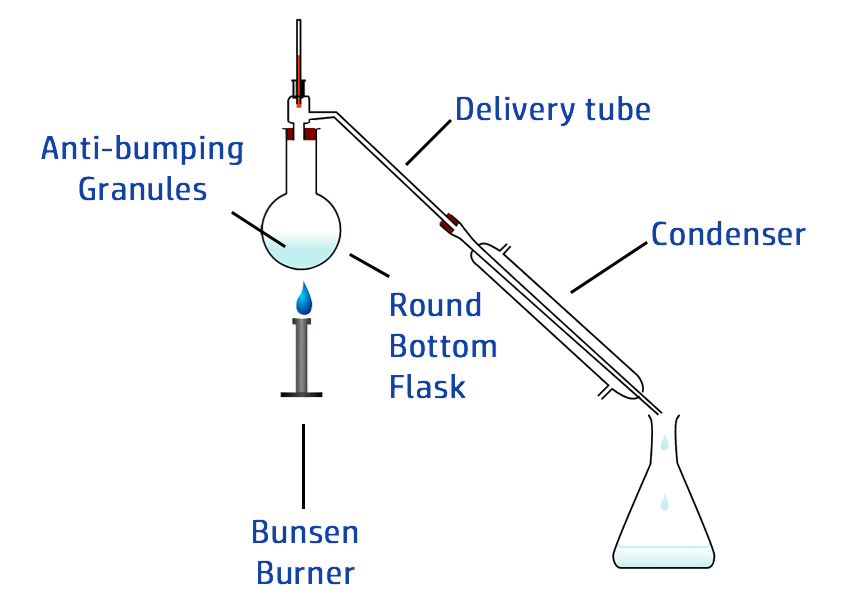
CH102: Describe how to carry out simple distillation
Simple distillation is a technique used to separate two different liquids based on their boiling points.
Example: Separating water (boils at 100oC) and ethanol (boils at 78oC).
- Heat the solution in a round bottom flask.
- Once it reaches 78oC, the ethanol will evaporate.
- The gas will rise and move into the condenser.
- The condenser is surrounded by cold water, which cools the gas down – turning it back into a liquid.
- If you stay below 100oC, the water will not evaporate.
CH103: Describe how to carry out fractional distillation
Fractional distillation is used to separate a mixture that contains lots of different liquids.
As the mixture is heated, a temperature gradient will exist – where it is hotter at the bottom of the fractionating column and cooler at the top.
Once the top of the column reaches the lowest boiling point – the first gas will move into the condenser and can be collected.
As the column continues to warm up, more and more of the fractions will move into the condenser, allowing them all to be collected separately.
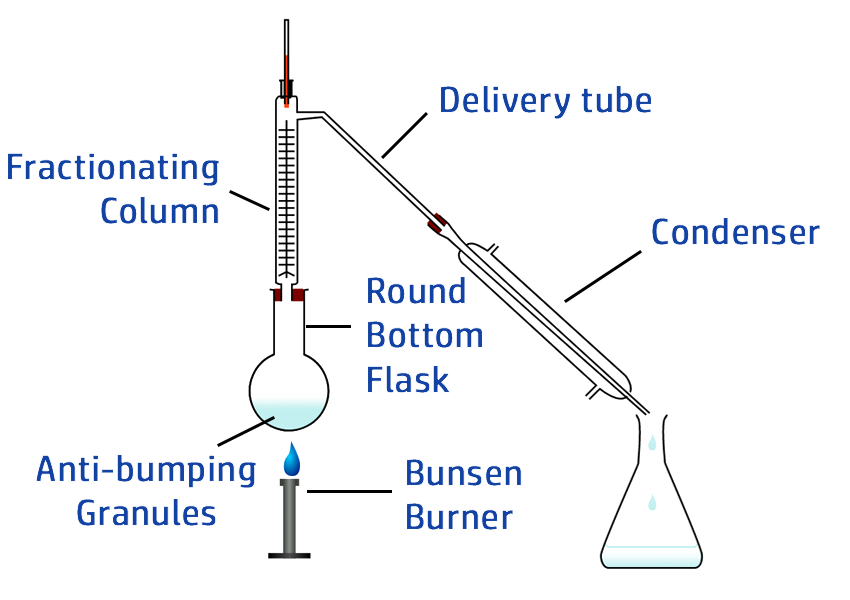
CH104: Suggest a suitable method to separate mixtures
CH105: Core Practical: Investigating Inks
Ink is a mixture of coloured substances dissolved in a liquid solvent. Ink can contain many different substances with different colours.
There are two types of ink – permanent ink and washable ink.
- Permanent ink cannot be washed and will not be separated when added to water.
- Washable ink can be separated when added to water.
If you are given an ink, there are two things you can do to analyse them.
Chromatography:
- Put a line in pencil (which is insoluble) on the bottom of the chromatogram.
- Add your sample(s) to the crosses.
- Once done, add the chromatogram to water making sure the ink does not touch the water. (If it does, the ink will dissolve and not move up the paper!)
- Once the water has travelled up the paper, remove and mark where the water reached.
If your ink does not move, it is insoluble (permanent).
If it does move, then one colour means it is pure, and more than one colour means it contains different colours.
Work out the retention factor for each ink (distance ink has moved ÷ distance water has moved). The number closes to 1 is the most soluble.
Simple Distillation:
- Heat the solution gently until it starts to boil.
- The ink with the lowest boiling point will start to evaporate and move into the delivery tube.
- Make sure you don’t heat the ink too much or the other colours may start to boil.
- Once the gas goes into the boiling tube, it will be surrounded by ice, cool and condense.
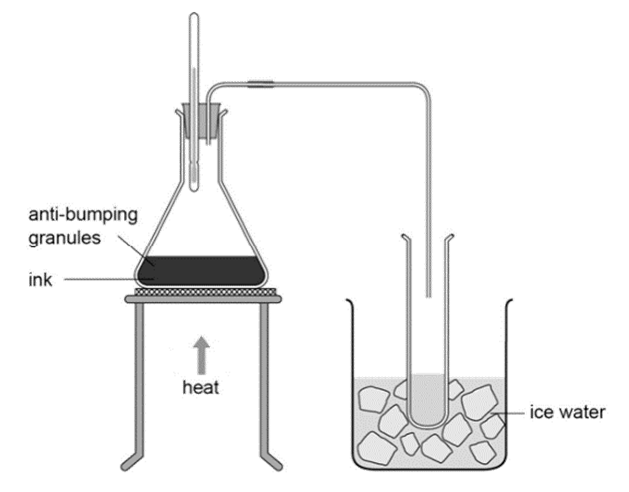
If the colour of the ink in the boiling tube is different than the colour of the original ink, it is a mixture of more than one colour.
If the ink in the boiling tube is the same colour as the ink left in the original flask, it is not a mixture – it is pure.
Hazards, risks, and precautions
A hazard something that can cause harm or damage something. The risk is how likely it is to cause harm.
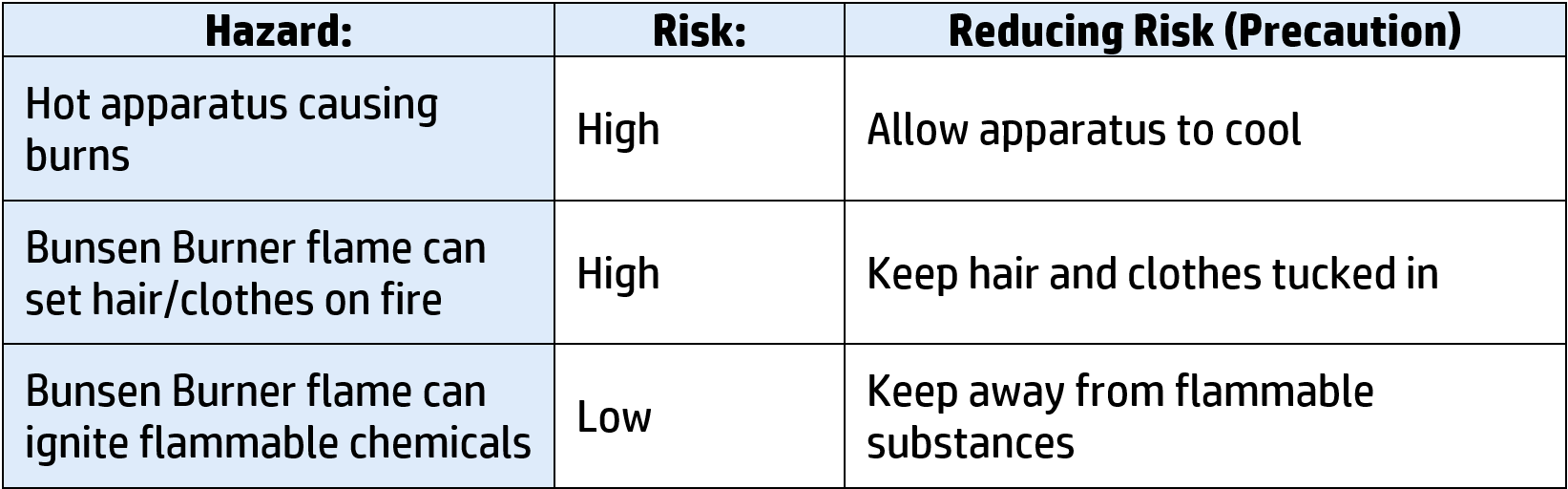
CH106: Describe to make salt water potable (safe to drink)
The word potable means safe to drink. This is how to make saltwater potable:
- Salt water is added to a large container and heated using crude oil.
- The water will evaporate and then hit the lid and cool back down – condensing it back down into water.
This use a lot of energy, so is not used to purify water on a large scale.
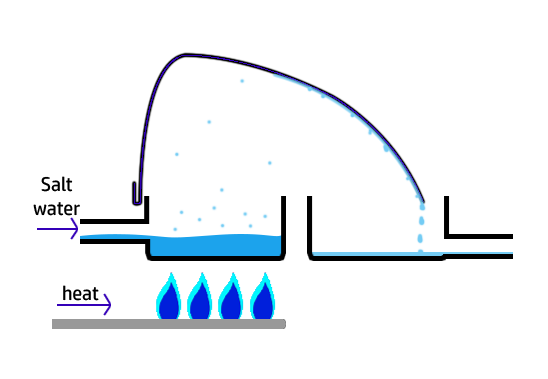
CH107: Describe how to make lake water potable (safe to drink)
To make water from lakes, reservoirs, or aquifers (underground rocks that contain water) potable, which means safe to drink, there are a series of steps the water must go through:
- Step 1: Screening. A sieve is used to remove large impurities, such as twigs and leaves.
- Step 2: Sedimentation. The water is left so that the small particles settle out at the bottom.
- Step 3: Filtration. Beds of sand and gravel are used to remove smaller insoluble particles that remain.
- Step 4: Chlorination: Chlorine is added to sterilise the water – killing any microorganisms present.
CH108: Explain why we use deionised water during chemical analysis
It is important to use pure distilled/deionised water (which don’t contain any dissolved salts) during chemical analysis because:
- It could give off incorrect results or hide correct results.
- It could result in cloudy precipitates being formed.
- It could lead to an incorrect conclusion from chemical analysis
Pure Water only contains H2O – there are no minerals in it, which are present in normal tap water.